Modified polyethylene naphthalate containing imide structure and preparation method thereof
A technology of polyethylene naphthalate and dimethyl naphthalate, applied in the field of polymer materials and their synthesis, can solve the problems of small processing temperature range, oxidation of polyester main chain, etc. The effect of improving performance and glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
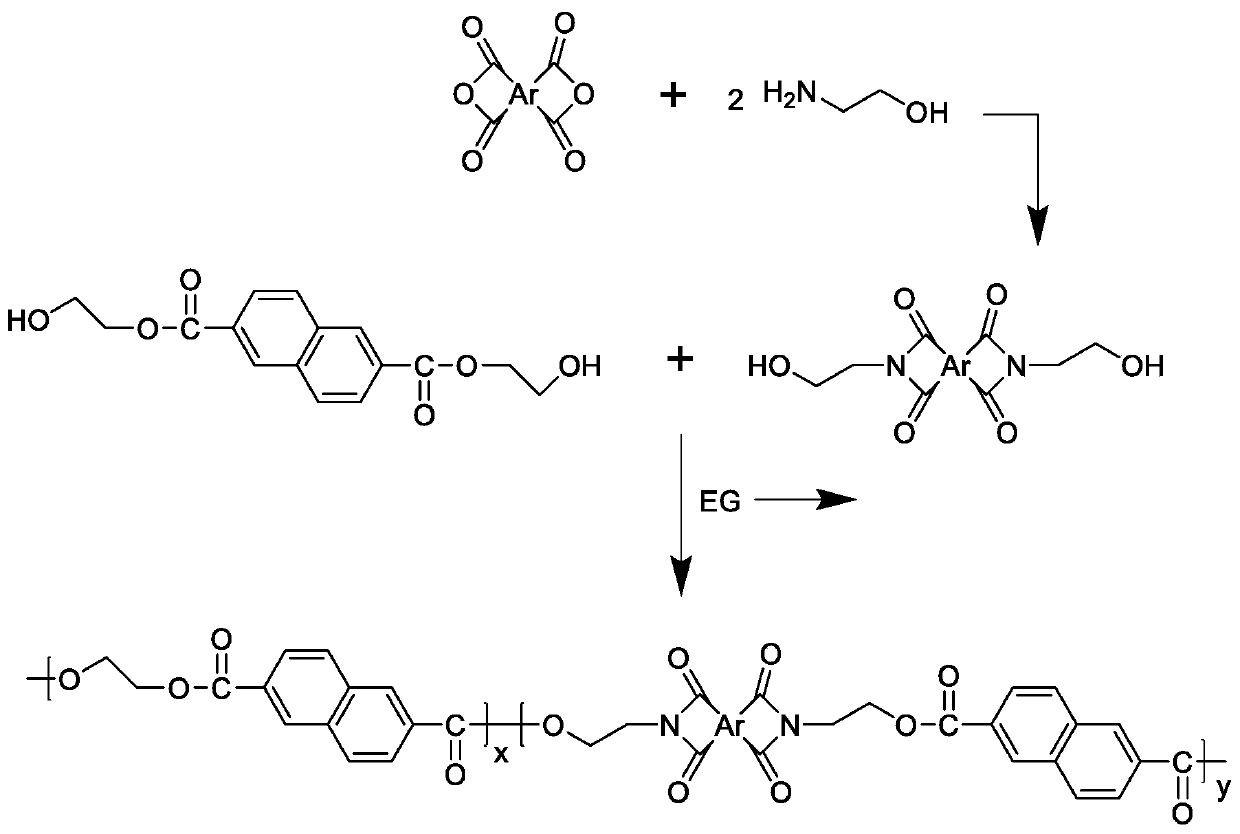
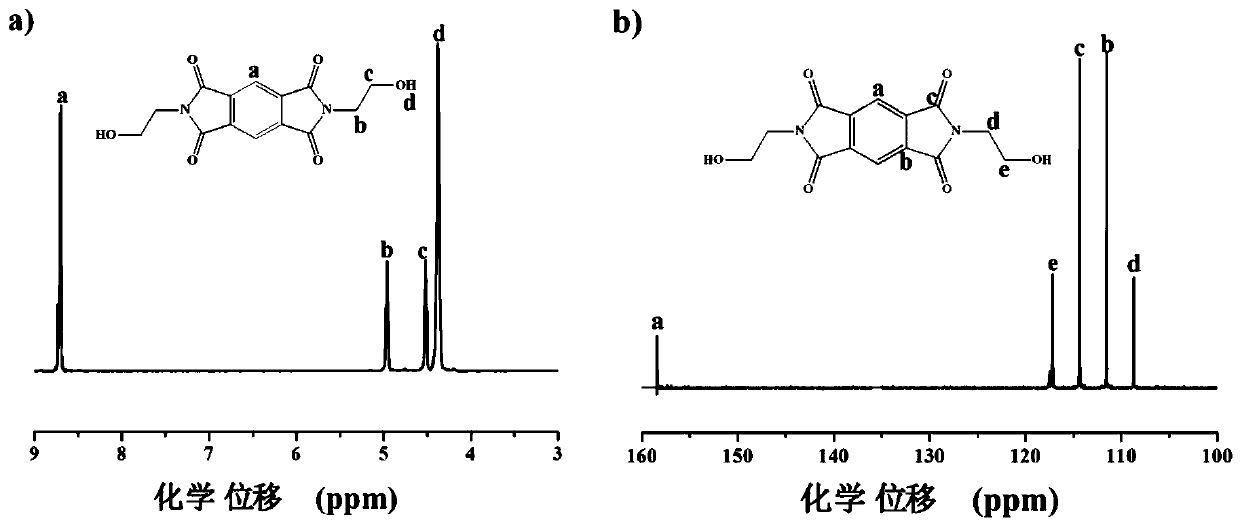
[0039] Embodiment 1: the synthesis of formula (III) type monomer, taking pyromellitic dianhydride as an example, get 218 grams (1 mole) pyromellitic dianhydride and 122 grams (2 moles) ethanolamine, dissolve in 4 liters of DMAc In the solution, add 306 grams (3 moles) of acetic anhydride after complete dissolution; react for 8 hours under nitrogen protection at a temperature of 140°C; cool to room temperature after the reaction, and then pour the solution into sufficient distilled water to obtain a yellow The solid precipitate is filtered, washed and dried to obtain N,N'-dihydroxyethyl-pyromellitic diimide monomer derived from pyromellitic dianhydride;
[0040]
[0041] Derived from 3,3',4,4'-biphenyltetracarboxylic dianhydride, 4,4'-biphenyl ether dianhydride, 3,3',4,4'-benzophenonetetracarboxylic dianhydride, 1, The dihydroxyethyl imide monomer of 4,5,8-naphthalene tetracarboxylic dianhydride can be prepared according to similar synthesis conditions and steps.
Embodiment 2
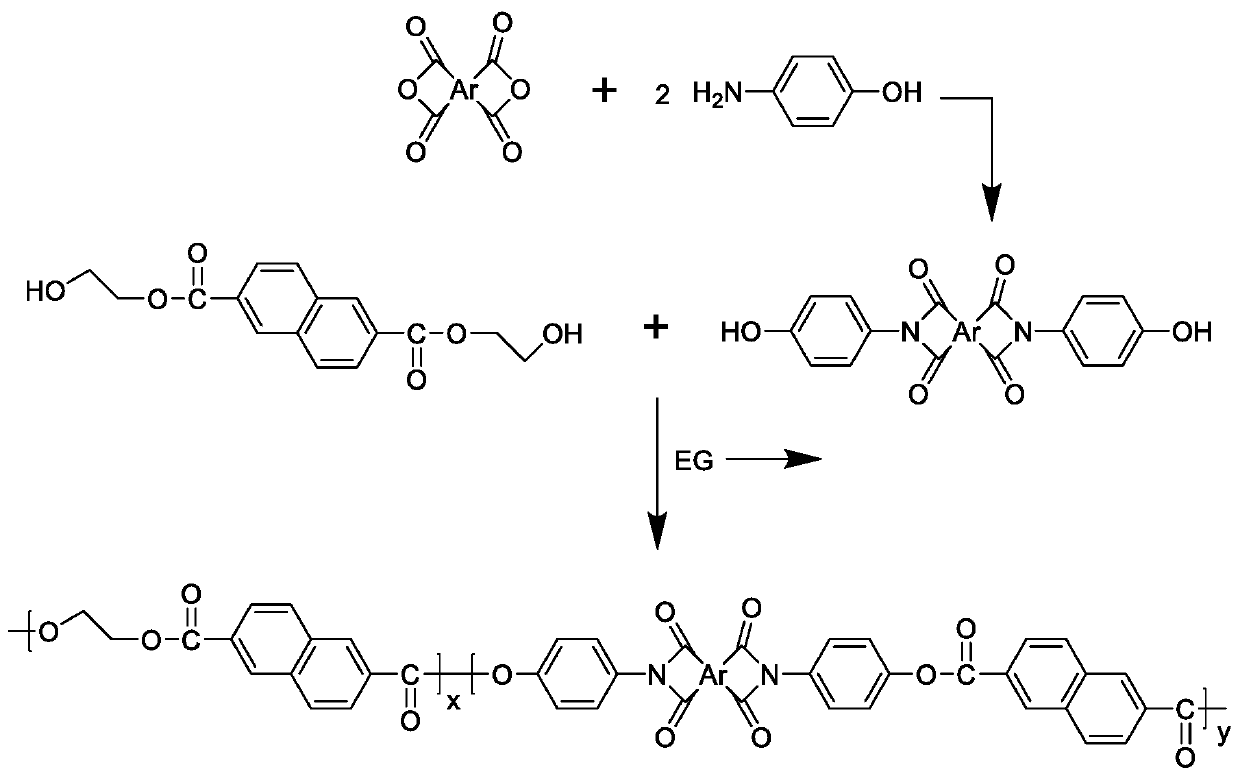
[0042] Embodiment 2: the synthesis of formula (IV) class monomer, take pyromellitic dianhydride as example
[0043] Get 218 grams (1 mole) pyromellitic dianhydride and 218 grams (2 moles) p-aminophenol, dissolve in 5 liters of DMAc, add 306 grams (3 moles) acetic anhydride after completely dissolving; Under nitrogen protection, React for 10 hours at a temperature of 150°C; after the reaction, cool to room temperature, then pour the solution into a sufficient amount of distilled water to obtain an orange solid precipitate, which is filtered, washed, and dried to obtain pyromellitic dianhydride N,N'-bis(4-hydroxyphenyl)-pyromellitic acid diimide monomer;
[0044]
[0045] Derived from 3,3',4,4'-biphenyltetracarboxylic dianhydride, 4,4'-biphenyl ether dianhydride, 3,3',4,4'-benzophenonetetracarboxylic dianhydride, 1, The bis(4-hydroxyphenyl)imide monomer of 4,5,8-naphthalene tetracarboxylic dianhydride can be prepared according to similar synthesis conditions and steps.
Embodiment 3
[0047] Add 91.275 grams of N,N'-dihydroxyethyl-pyromellitic acid diimide provided in Example 1, 36.636 grams of dimethyl 2,6-naphthalene dicarboxylate and 0.028 grams of anhydrous zinc acetate into the reactor 1; transesterification reaction under nitrogen protection, 195 °C for 4 hours to form 2,6-hydroxyethyl naphthalene dicarboxylate containing pyromellitic diimide structure; 207.604 grams of 2,6-naphthalene diimide Add dimethyl formate, 105.4 grams of ethylene glycol and 0.156 grams of anhydrous zinc acetate to reactor 2; react under nitrogen protection and 195°C for 3 hours to form ethylene glycol 2,6-naphthalene dicarboxylate, and then Add it into the reactor 1; gradually raise the temperature of the reactor 1 to 295 ° C, and react for 1 hour under the condition of a vacuum degree of less than 100 Pa to completely remove the by-product ethylene glycol; stop heating the reactor after the reaction, and pass nitrogen gas to make the reaction The pressure of the container is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com