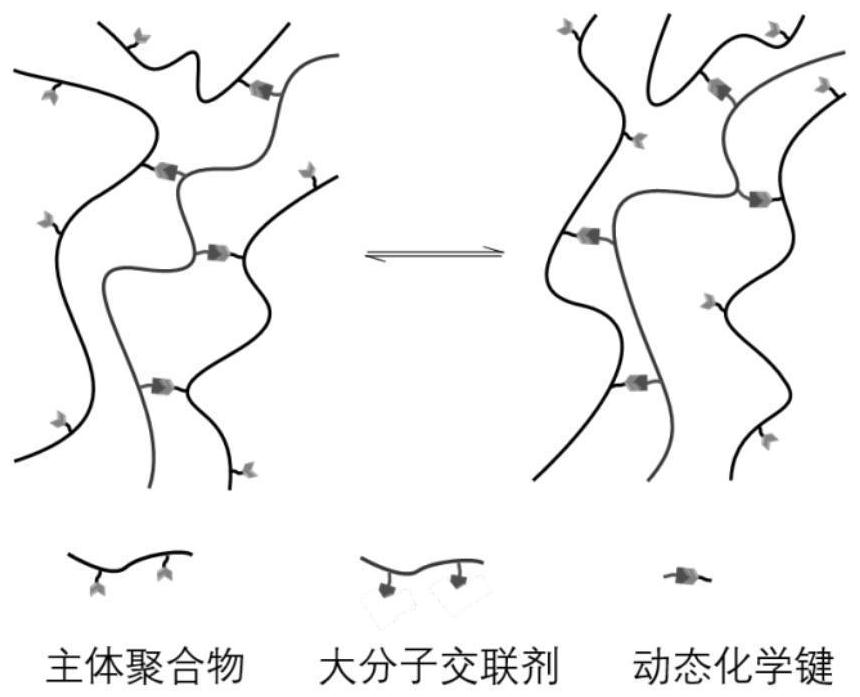
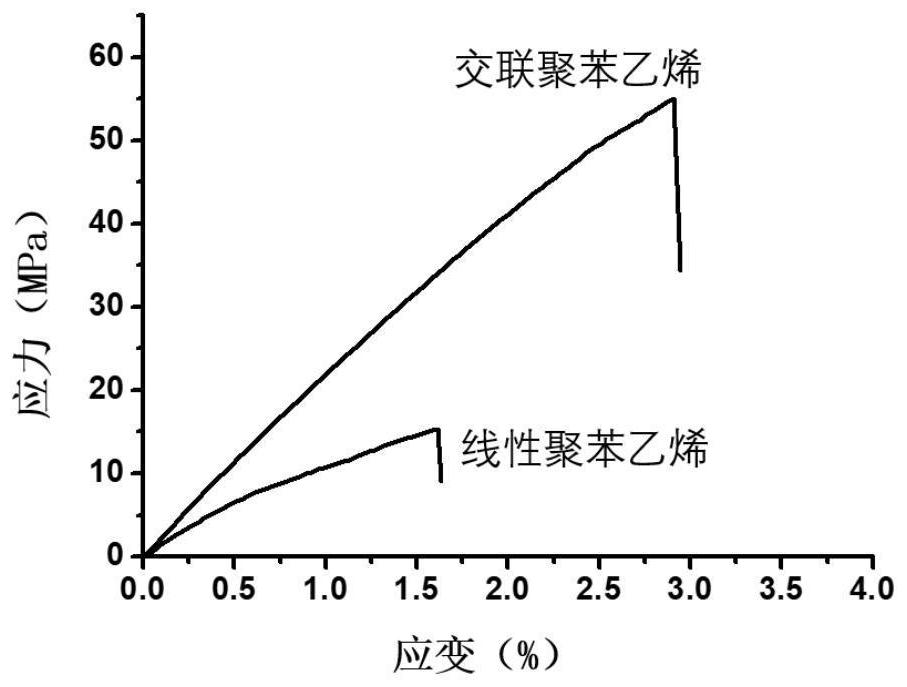
A Synthetic Method for Reproducible Thermoset Polymers
A technology of repeated processing and synthesis method, which is applied in the synthesis field of thermosetting polymers, can solve the problems of poor tolerance to organic solvents, pollution, organic solvent corrosion, etc., and achieve the effect of improving mechanical properties, simple synthesis process, and good operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Synthesis of repeatable crosslinked styrene (a method of synthesizing a thermosetting polymer thereof) of styrene and its derivative monomer.
[0038] Styrene 100 mmol, monomer A (glycidyl methacrylate), 0.1 mmol, and azo diisobutyronitrile 0.02 mmol. 10 ml of dimethyl sulfoxide was added, and then the reaction bottle was stirred in an oil bath at 110 ° C for 24 hours, then the reaction was cooled to room temperature, and the reaction liquid was poured into methanol precipitation, vacuum dried to constant weight. To obtain a polymer 1. Its synthetic route is:
[0039]
[0040] Styrene 10 mmol is added to the Schlenk reaction flask, monomer B (vinylbenzyl bio), RAFT initiator 0.01 mmol, azo diisobutyronitrile 0.002 mmol. 2 mL of dimethyl sulfoxide was added, then the reaction bottle was stirred in an oil bath at 110 ° C for 24 hours, then the reaction was cooled to room temperature, and the reaction liquid was poured into methanol precipitation, vacuum drying to ...
Embodiment 2
[0045] Example 2, Synthesis of Methyl Crosslinked Polymethyl methacrylate (a method of synthesizing thermosetting polymers representing a methacrylate monomer).
[0046] Methyl methacrylate 100 mmol, monomer A (glycidyl methacrylate), 0.1 mmol, and azo diisobutyronitrile 0.02 mmol. 10 ml of dimethyl sulfoxide was added, and then the reaction bottle was stirred in an oil bath at 65 ° C for 16 hours, then the reaction was cooled to room temperature, and the reaction liquid was poured into methanol precipitation, vacuum dried to constant weight. To obtain a polymer 4. Its synthetic route is:
[0047]
[0048] Methyl methacrylate 10 mmol, monomer B (vinyl bio), 0.002 mmol of Azobimobutyronitrile 0.002 mmol. 2 ml of dimethyl sulfoxide was added, and then the reaction bottle was stirred in an oil bath at 65 ° C for 16 hours, then the reaction was cooled to room temperature, and the reaction liquid was poured into methanol precipitation, vacuum dried to constant weight. To obtain a pol...
Embodiment 3
[0053] Example 3, synthesis of a repeatable crosslinked acrylate niutate (a method of synthesizing a thermosetting polymer representing an acrylate monomer).
[0054] A n-butyl acrylate 100 mmol, monomer A (glycidyl methacrylate), 0.1 mmol, azo diisobutyronitrile 0.02 mmol. 10 ml of dimethyl sulfoxide as a solvent was added, and then the reaction bottle was stirred in an oil bath at 65 ° C for 8 hours, then the reaction was cooled to room temperature and 10 ml of tetrahydrofuran was added, and the mixture was mixed into petroleum ether and precipitated. The vacuum is dried to constant weight to give the polymer 7. Its synthetic route is:
[0055]
[0056] N-acrylate 10 mmol, monomer B (vinyl bio), Raft initiator 0.01 mmol, 0.002 mmol of Azobiobutyrignonium. 2 ml of dimethyl sulfoxide was added, and then the reaction bottle was stirred in an oil bath at 65 ° C for 8 hours, and then the reaction was cooled to room temperature and 10 ml of tetrahydrofuran was added, and the mixed u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com