A kind of preparation method of the aryl acridine derivative synthesized by palladium catalysis
A technology of aryl acridine and its derivatives, which is applied in the field of preparation of aryl acridine derivatives, can solve the problems of incapability of real large-scale promotion, limitations of practicability and applicability, and non-compliance with the development of green chemistry, etc. The effect of healthy operators, strong substrate universality, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
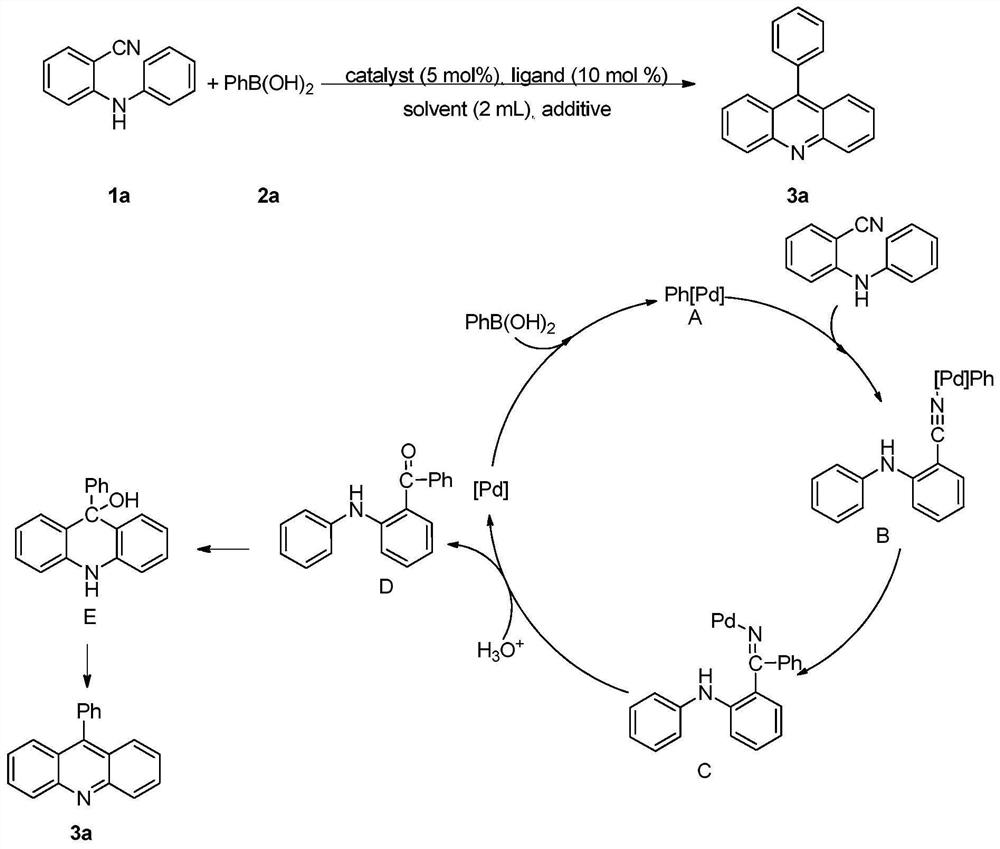
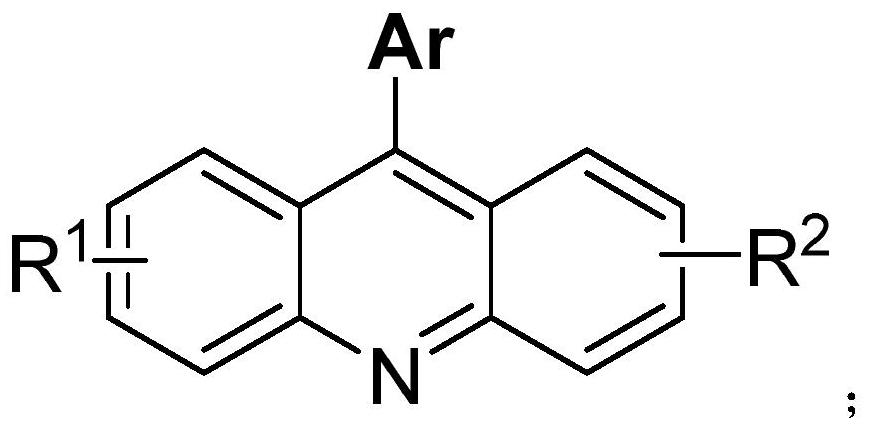
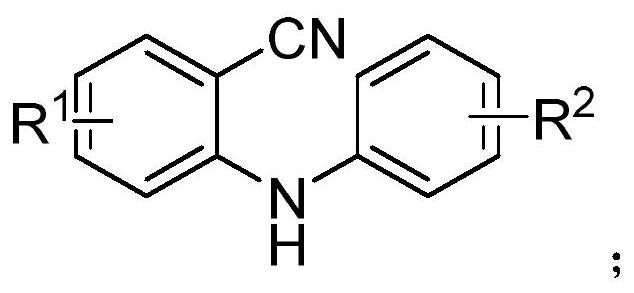
[0032] Under air atmosphere, add raw material 2-(phenylamino) benzonitrile (0.5mmol), phenylboronic acid (0.75mmol), palladium catalyst palladium acetate (5mol%), 2,2'-bipyridine ( 6mol%), methanesulfonic acid (1.0mmol) and solvent water (2mL) were stirred and mixed; after mixing uniformly, reacted at 100°C for 24h to obtain 9-phenylacridine; the product yield was 94% ;
[0033] Characterization data: 1 H NMR (400MHz, CDCl 3 )δ8.34(d, J=8.8Hz, 2H), 7.82(t, J=7.9Hz, 2H), 7.77-7.75(m, 2H), 7.76-7.63(m, 3H), 7.50-7.45(m ,4H); 13 C NMR (100MHz, CDCl 3 )δ148.8, 147.2, 136.0, 130.4, 130.0, 129.6, 128.5, 128.4, 126.9, 125.6, 125.2.9 The structural formula of phenylacridine is
[0034]
[0035] Its reaction formula is
[0036]
Embodiment 2
[0038] Under air atmosphere, add raw materials 2-(phenylamino)benzonitrile (0.5mmol), 3-chlorophenylboronic acid (0.75mmol), palladium catalyst palladium acetate (5mol%), ligand 2,2 `-Bipyridine (6mol%), additive methanesulfonic acid (1.0mmol) and solvent water (2mL) are stirred and mixed; After mixing evenly, react 24h under the condition of 100 ℃, make 9-(3-chlorobenzene base) acridine; the yield of the final product was 96%. Characterization data:
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.30(d, J=8.8Hz, 2H), 7.81-7.77(m, 2H), 7.67(d, J=8.7Hz, 1H), 7.48-7.44(m, 3H), 7.36-7.32(m ,1H); 13 C NMR (125MHz, CDCl3) δ148.8, 145.6, 130.8, 134.0, 134.8, 130.6, 130.3, 130.0, 129.8, 128.8, 127.8, 126.6, 126.2, 125.1.
[0040] The structural formula of 9-(3-chlorophenyl)acridine is as follows:
[0041]
[0042] Its reaction formula is:
[0043]
Embodiment 3
[0045] Under air atmosphere, add raw material 2-(phenylamino)benzonitrile (0.5mmol), 4-methoxyphenylboronic acid (0.75mmol), palladium catalyst palladium acetate (5mol%), ligand 2 , 2`-bipyridine (6mol%), additive methanesulfonic acid (1.0mmol) and solvent water (2mL) are stirred and mixed; after mixing evenly, react 24h under the condition of 100 ℃, obtain 9-(4- Methoxyphenyl) acridine; the yield of the final product was 90%.
[0046] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ8.27(d, J=8.9Hz, 2H), 7.77-7.76(m, 4H), 7.13(d, J=8.3Hz, 2H), 3.94(S, 3H); 13 C NMR (125MHz, CDCl 3 )δ159.9, 149.1, 147.3, 131.9, 130.0, 129.8, 128.2, 127.1, 125.7, 125.6, 114.1, 55.6.
[0047] The structural formula of 9-(4-methoxyphenyl)acridine is as follows:
[0048]
[0049] Its reaction formula:
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com