Method for extracting lithium resource from salt lake brine through electric flocculation
A technology of salt lake brine and electric flocculation, which is applied in the direction of lithium carbonate; Good, high extraction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
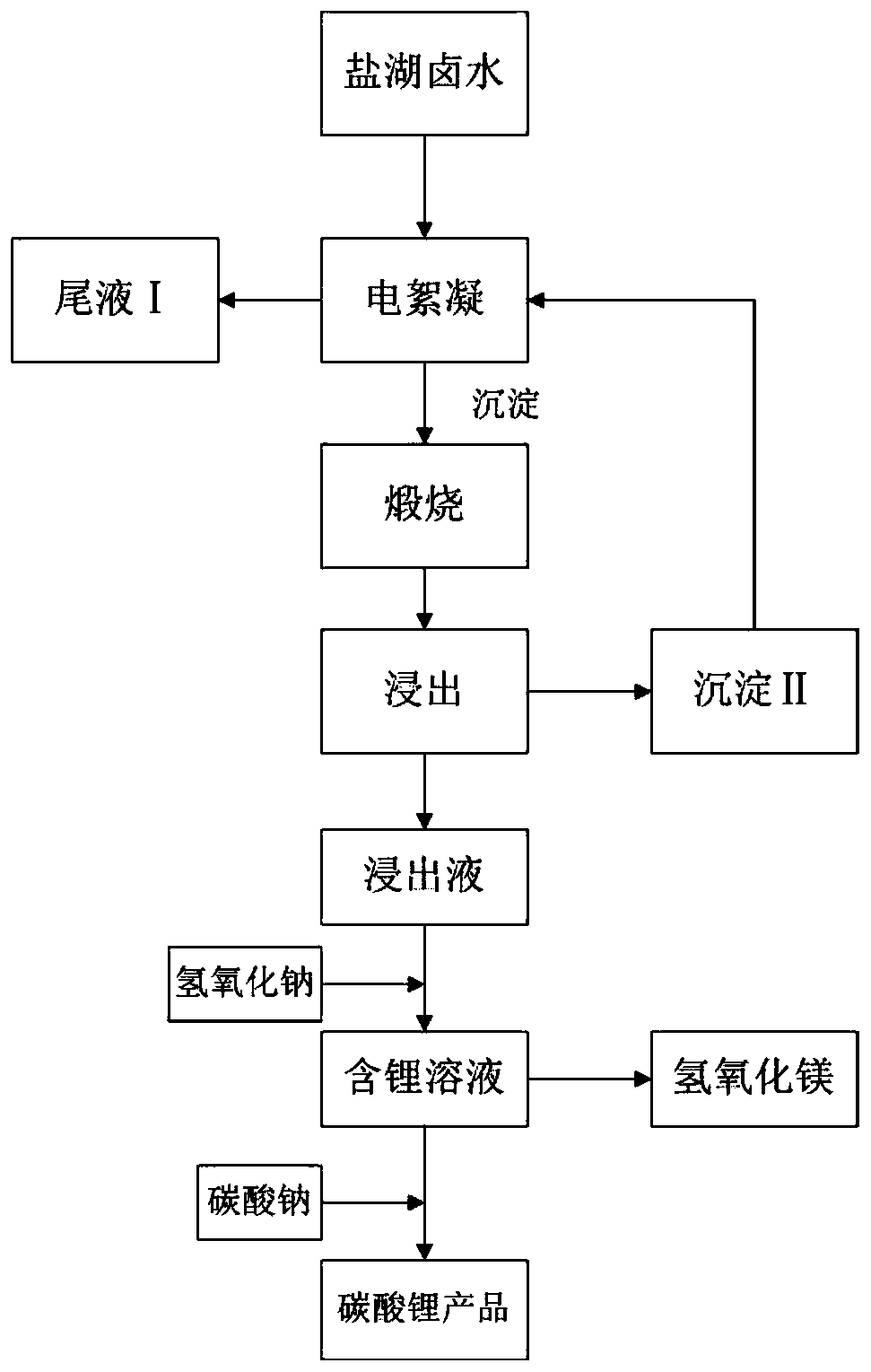
[0031] (1) Select a salt lake brine with a high magnesium-lithium ratio in Qinghai. The lithium content in the brine is 0.28g / L, the magnesium content is 12.76g / L, and the magnesium-lithium ratio is 45.6. The anode plate of the electroflocculation device is an aluminum plate, and the cathode plate For aluminum plate, the current density is 80mA / cm 2 , the pH is 5.6, the distance between the plates is 1cm, the stirring intensity is 750r / min, after electrolysis reaction for 150min, the tail liquid I and precipitate are obtained by solid-liquid separation.
[0032] (2) The precipitate in step (1) was calcined at 450°C for 30min, and the calcined product was leached for 30min at 40°C with a liquid-solid ratio of 5:1, and separated by filtration to obtain alumina slag and lithium-containing solution.
[0033] (3) in the lithium-containing solution in step (2), after testing, still contain the magnesium impurity of 2.5g / L, add NaOH in the lithium-containing solution, the add-on of N...
Embodiment 2
[0036] (1) Select a salt lake brine with a high magnesium-lithium ratio in Xinjiang. The content of lithium in the brine is 2.1g / L, the content of magnesium is 113.6g / L, and the ratio of magnesium to lithium is 54.1. The anode plate of the electrocoagulation device is an aluminum plate, and the cathode plate For the iron plate, the current density is 150mA / cm 2 , the pH is 6, the distance between the plates is 1.5cm, the stirring intensity is 800r / min, after electrolysis for 180min, the tail liquid I and the precipitate are obtained by solid-liquid separation.
[0037] (2) The precipitate in step (1) was calcined at 400°C for 50min, and the calcined product was leached for 50min at 60°C with a liquid-solid ratio of 8:1, and separated by filtration to obtain alumina slag and lithium-containing solution.
[0038](3) in the lithium-containing solution in step (2), after testing, still contain the magnesium impurity of 3.3g / L, add NaOH in the lithium-containing solution, the add-o...
Embodiment 3
[0041] (1) Select a salt lake brine with a high magnesium-lithium ratio in Tibet. The lithium content in the brine is 1.5g / L, the magnesium content is 60g / L, and the magnesium-lithium ratio is 40. The anode plate of the electroflocculation device is an aluminum plate, and the cathode plate is Graphite plate with a current density of 115mA / cm 2 , the pH is 7, the distance between the plates is 1cm, the stirring intensity is 700r / min, after electrolysis reaction for 150min, the tail liquid I and precipitate are obtained by solid-liquid separation.
[0042] (2) The precipitate in step (1) was calcined at 500°C for 40 minutes, and the calcined product was leached for 30 minutes at 50°C with a liquid-solid ratio of 10:1, and separated by filtration to obtain alumina slag and lithium-containing solution.
[0043] (3) In the lithium-containing solution in step (2), after testing, still contain the magnesium impurity of 3g / L, add NaOH in the lithium-containing solution, the add-on of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com