Preparation method of micafungin derivative side chain intermediate
A technology of micafungin and derivatives, applied in the field of medicine and chemical industry, can solve the problems of high difficulty in industrial production and application, complex process, environmental pollution, etc., and achieve the effects of avoiding a large amount of acid and alkali waste water, shortening the process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
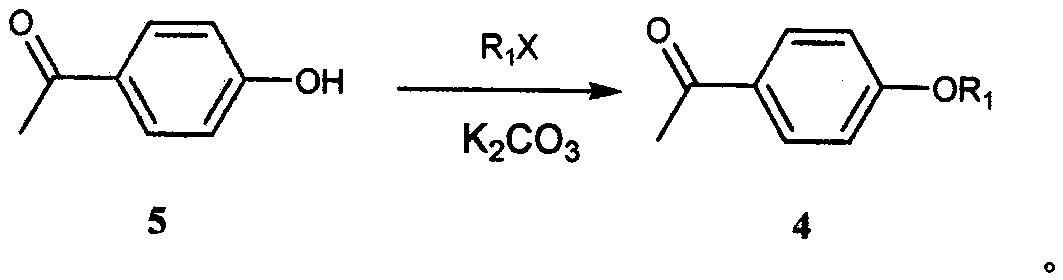
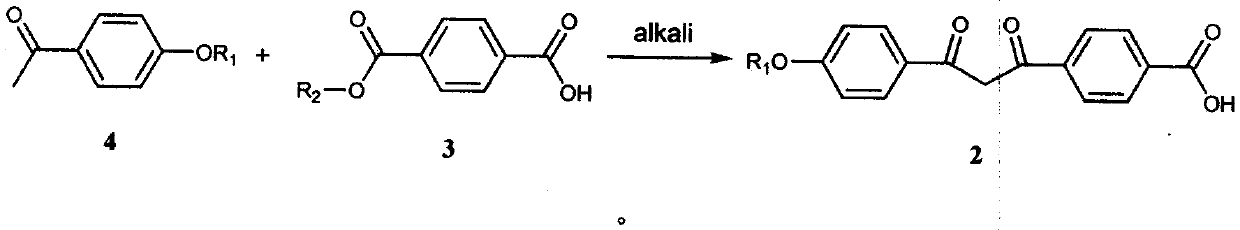
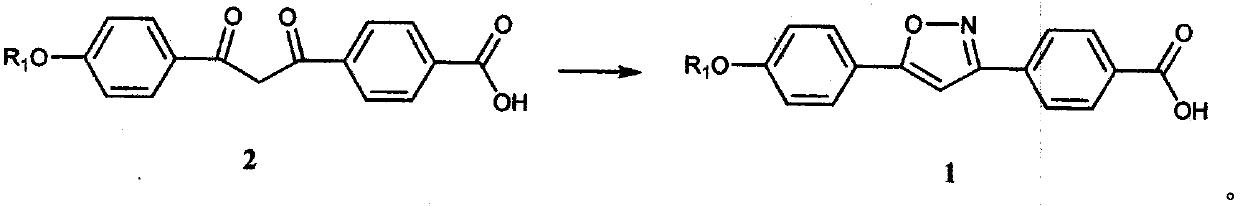
[0041] Add 35.0g of p-hydroxyacetophenone, 46.6g of 1-bromopentane, 71.0g of anhydrous light potassium carbonate, and 175ml of N,N-dimethylformamide into a 500ml reaction flask in sequence, and store at 60°C under nitrogen protection The reaction was stirred at ~80°C for 5 hours. After the reaction was completed, it was quenched with 250ml of purified water, and then extracted three times with 350ml of n-hexane. The organic layers were combined and the organic solvent was removed by rotary evaporation to obtain an oil (Formula 4). Put all the oil, 55.5g of monomethyl terephthalate, 86.5g of potassium tert-butoxide and 350ml of N,N-dimethylformamide into a 1000ml reaction bottle in sequence, and stir at 10℃~30℃ under the protection of nitrogen React for 5 hours, quench the reaction with 1L of methanol, adjust the pH to 4-5 with concentrated hydrochloric acid, vacuum filter to dryness, wash the filter cake with 350ml of methanol and 500ml of purified water, and dry the filter cak...
Embodiment 2
[0044] Add 35.0g of p-hydroxyacetophenone, 54.8g of 1-chloropentane, 42.6g of anhydrous light potassium carbonate, and 175ml of N,N-dimethylformamide into a 500ml reaction bottle in sequence, and keep it under nitrogen protection at 60°C The reaction was stirred at ~80°C for 4 hours, quenched with 250ml of purified water after the reaction was completed, then extracted three times with 350ml of n-hexane, combined the organic layers and rotary evaporated to remove the organic solvent to obtain an oily substance (Formula 4). Put all the oil, 59.8g of monoethyl terephthalate, 74.2g of sodium tert-butoxide and 350ml of N,N-dimethylformamide into a 1000ml reaction bottle in turn, and stir at 10°C to 30°C under nitrogen protection React for 5 hours, quench the reaction with 1L of methanol, adjust the pH to 4-5 with concentrated hydrochloric acid, vacuum filter to dryness, wash the filter cake with 350ml of methanol and 500ml of purified water, and dry the filter cake in vacuum at 60°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com