Application of boron carbonitride material in catalyzing oxidative dehydrogenation of light alkane to prepare olefin
A low-carbon alkane and oxidative dehydrogenation technology, which is applied in the direction of hydrocarbons, hydrocarbons, physical/chemical process catalysts, etc., can solve the problems of high cost and complicated preparation process of boron nitride materials, and achieve low cost and excellent The effect of versatility and good olefin yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The application of the boron, carbon and nitrogen materials described in this example in the preparation of alkenes by catalyzing the oxidative dehydrogenation of light alkanes includes the following steps:
[0022] 1) Place 0.1g of boron carbon nitrogen material in a quartz tube in a fixed-bed reactor, and raise the temperature to 450°C at 5°C / min under a mixed atmosphere of propane, oxygen, and helium; the volume of propane, oxygen, and helium The ratio is 1:1:4, and helium plays a diluting role;
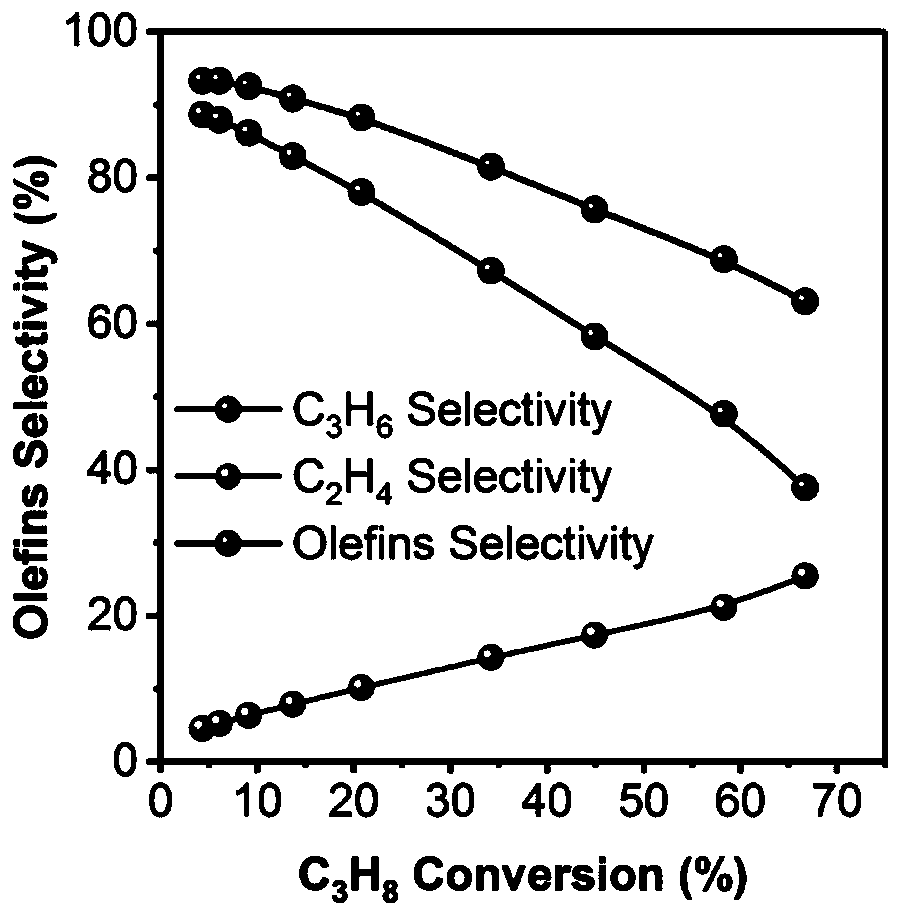
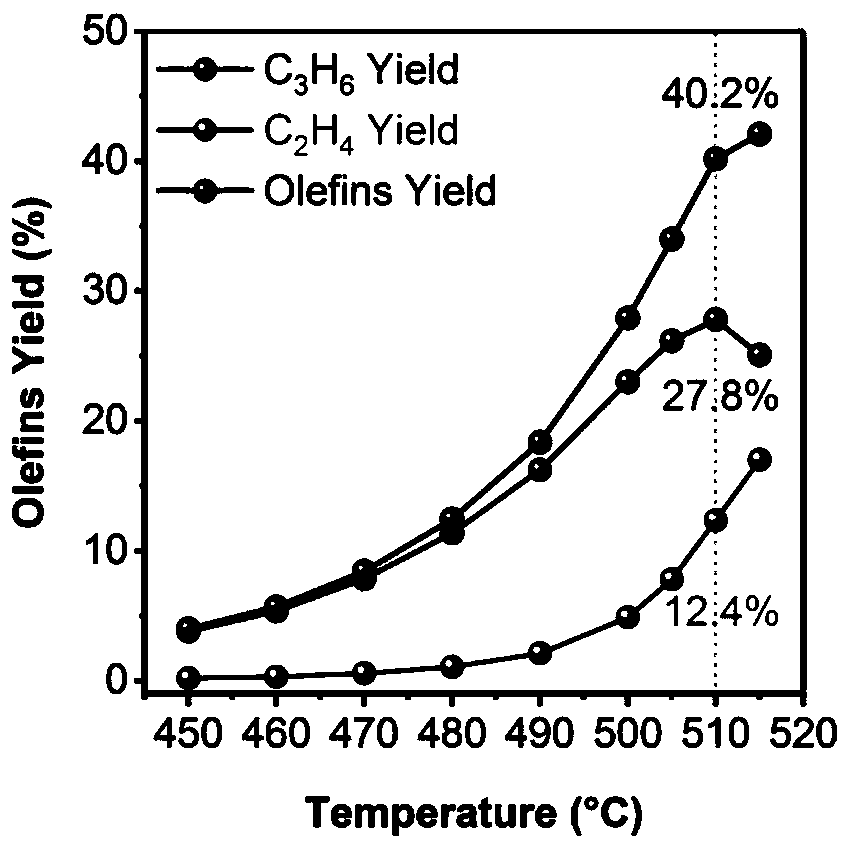
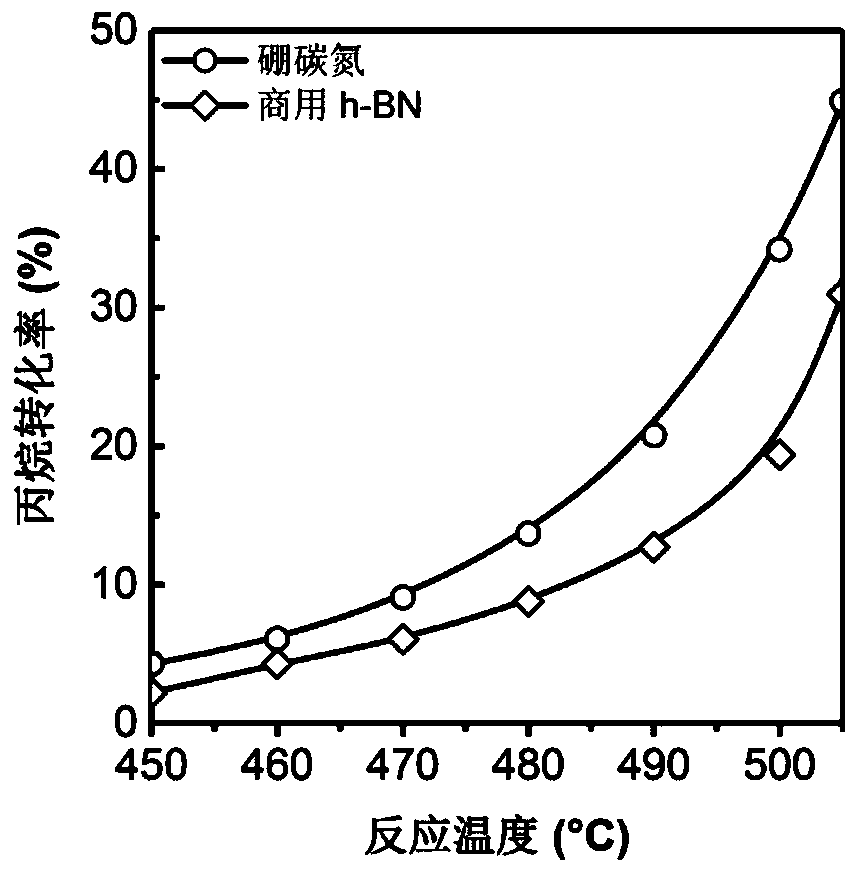
[0023] 2) After the temperature in step 1) is stabilized for half an hour, use the online chromatographic test system to start the test. The total flow rate of the mixed gas is 36ml / min, the reaction temperature is 450-520°C, and the pressure is 0.1MPa. After stabilizing for half an hour, Start to collect data, and the test results are: when the reaction temperature is 450°C, the conversion rate of propane is 4.3%, and the selectivity of propylene is 89%; when the reaction ...
Embodiment 2
[0025] The application of the boron, carbon and nitrogen materials described in this example in the preparation of alkenes by catalyzing the oxidative dehydrogenation of light alkanes includes the following steps:
[0026] 1) Place 0.3g of boron carbon nitrogen material in a quartz tube in a fixed bed reactor, and raise the temperature to 500°C at 10°C / min under the protection of oxygen, ethane and helium, in which ethane, oxygen and helium The volume ratio is 2:1:4, and the helium plays a diluting role;
[0027] 2) After the temperature in step 1) is stable, use the online chromatographic test system to start the test. The total flow rate of the mixed gas is 49ml / min, the reaction temperature is 500-600°C, and the pressure is 0.1MPa. After half an hour of stabilization, start to collect data, the test results are: the reaction temperature is 500°C, the conversion rate of ethane is 11%, and the selectivity of ethylene is 94%; and when the reaction temperature is 550°C, the con...
Embodiment 3
[0029] The application of the boron, carbon and nitrogen materials described in this example in the preparation of alkenes by catalyzing the oxidative dehydrogenation of light alkanes includes the following steps:
[0030] 1) Place 0.2g of boron carbon nitrogen material in a quartz tube in a fixed bed reactor, and raise the temperature to 430°C at 10°C / min under the protection of oxygen, butane and nitrogen, wherein the volume of butane, oxygen and nitrogen The ratio is 2:1:4, nitrogen plays a diluting role;
[0031] 2) After the temperature in step 1) is stable, use the online chromatographic test system to start the test. The total flow rate of the mixed gas is 42ml / min, the reaction temperature is 430-500°C, and the pressure is 0.1MPa. After half an hour of stabilization, every 10 The data is collected once at ℃, and then the changes of butane conversion and product selectivity are observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com