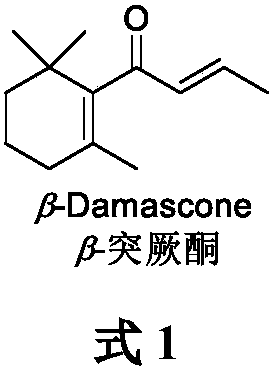
Synthesis process of beta-damascenone
A synthesis process, the technology of Turkone, applied in the field of industrial production of β-Turkone, can solve the problems of high cost, complicated operation, and difficulty in industrial production, and achieve low raw material cost, high product purity, and low energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
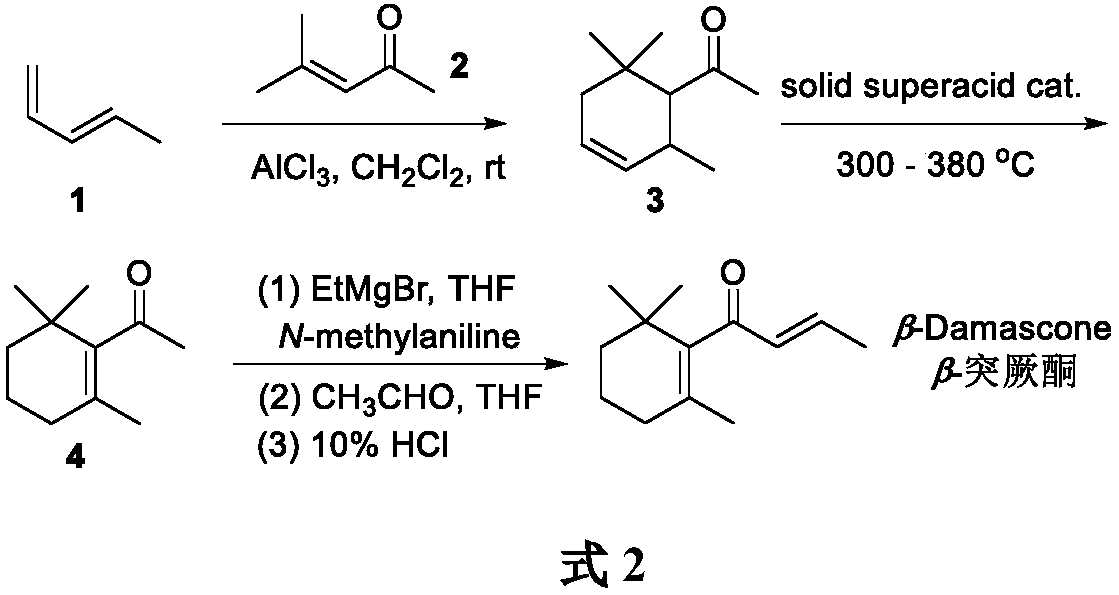
[0021] Synthesis of Diene to Prepare 1-(2,6,6,-Trimethylcyclohex-3-enyl)-ethanone
[0022] Add dichloromethane (200L) and anhydrous aluminum trichloride (319Kg, 2400mol) in the reaction kettle, stir well, add dropwise the dichloromethane of 4-methyl-3-penten-2-one (294Kg, 3000mol) Methane solution (150L), stirred for 2h, passed into 1,3-pentadiene, continued to stir the reaction, GC monitored the reaction, stopped the reaction when the content of 4-methyl-3-penten-2-one was lower than 1% . Ice water (400 L) was added to quench the reaction, and the layers were separated. The organic phase was washed with saturated aqueous sodium chloride solution, dichloromethane was recovered by distillation, and the remaining organic phase was sent to a rectification kettle for rectification to obtain 1-(2,6,6,-trimethylcyclohex-3-enyl )-ethanone (358Kg, yield 72%).
Embodiment 2
[0024] Preparation of 1-(2,6,6,-trimethylcyclohex-1-enyl)-ethanone by isomerization of olefins
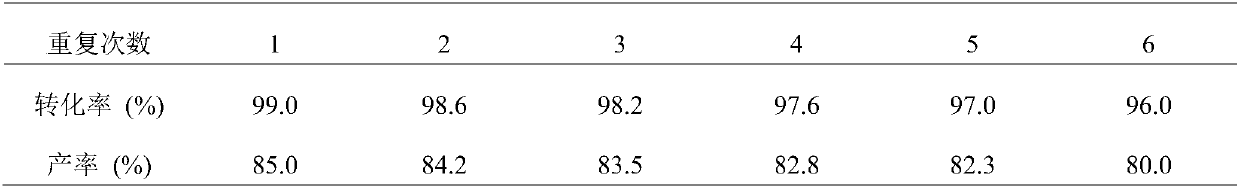
[0025] Will SO 4 2- / ZrO 2 Solid superacid catalyst (7.9Kg, 36mol) packs diameter 0.16 meters, in the gas-phase reactor of 5 meters in length, under nitrogen purge, gas-phase reactor is heated to 300 ℃, then depressurizes and makes gas-phase reactor internal pressure be 45mmHg, use a quantitative pump to introduce 1-(2,6,6,-trimethylcyclohex-3-enyl)-ethanone (60Kg, 360mol) into the gas phase reactor at a speed of 7.5Kg / h, Continue to maintain the reaction at 300-380°C. After the reaction is over, the reaction gas enters the cooling tower to cool, and the cooled mixture is sent to the rectification kettle for rectification to obtain 1-(2,6,6,-trimethylcyclohexane -1-enyl)-ethanone (51 Kg, 85% yield, 99% conversion, 95% purity). SO 4 2- / ZrO 2 The solid superacid catalyst continues to be recycled 6 times.
[0026] Table 1 The impact of catalyst recycling on conversion and yie...
Embodiment 3
[0029] Preparation of β-Turkone by Aldol Condensation
[0030]Add ethylmagnesium bromide tetrahydrofuran solution (1.0M, 1800L) into the reaction kettle, pass frozen brine into the cooling jacket of the reaction kettle, wait until the reaction kettle is cooled to 0°C, add N-methylaniline tetrahydrofuran dropwise The solution (128.5Kg, 1200mol, 500L) was stirred evenly at 0°C, and 1-(2,6,6,-trimethylcyclohex-1-enyl)-ethanone (166Kg, 1000mol) was added. The temperature of the reactor was raised to room temperature, and the stirring reaction was continued for 5 h. Slowly add tetrahydrofuran solution (39.6Kg, 900mol, 300L) of acetaldehyde, GC monitors reaction, when the content of 1-(2,6,6,-trimethylcyclohex-1-enyl)-ethanone is lower than 1 %, add 10% hydrochloric acid solution (1000L), continue to stir the reaction for 1h, and stop the reaction. The layers were separated, and the aqueous phase was extracted with ethyl acetate (1000 L×2). The organic phases were combined and was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com