An external gel patch for treating arthritis
A gel patch and arthritis technology, applied in the direction of fern/filamentous plant medical raw materials, anti-inflammatory agents, medical preparations with non-effective ingredients, etc., can solve the problems of inconvenient taking and large side effects, etc. Achieve the effect of reducing individual differences, high compliance, and good transdermal absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
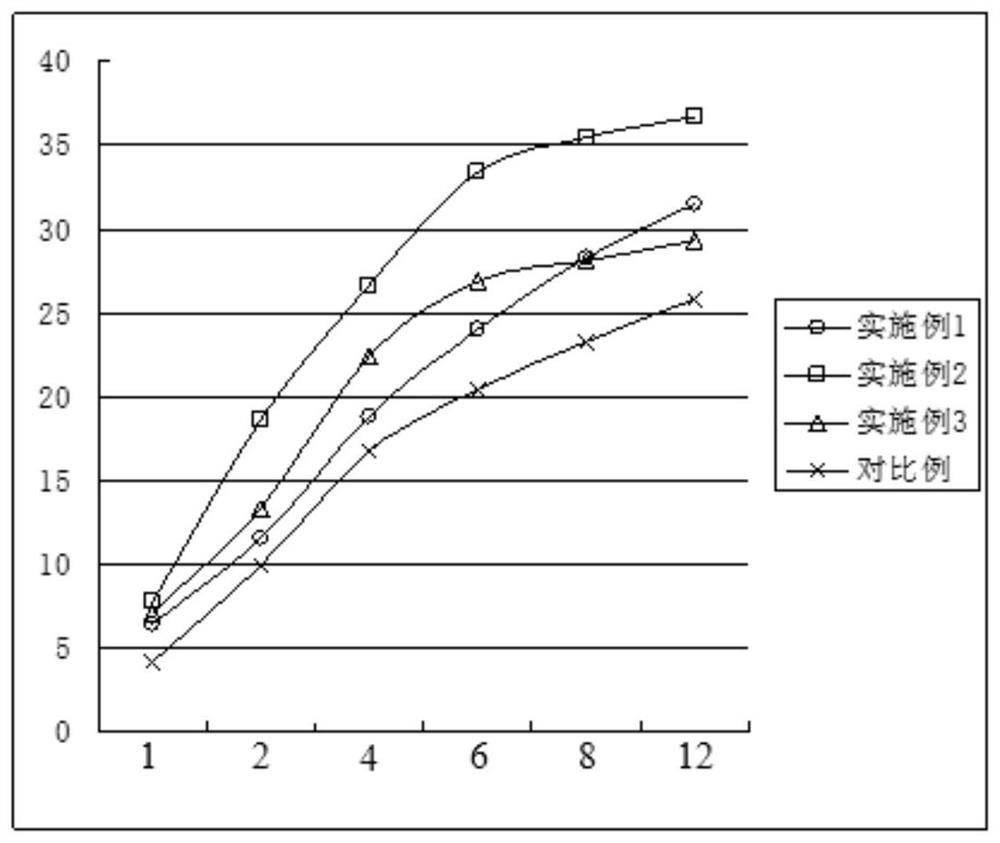
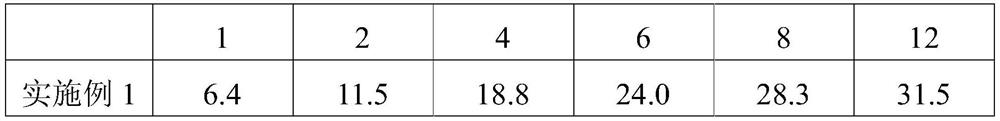
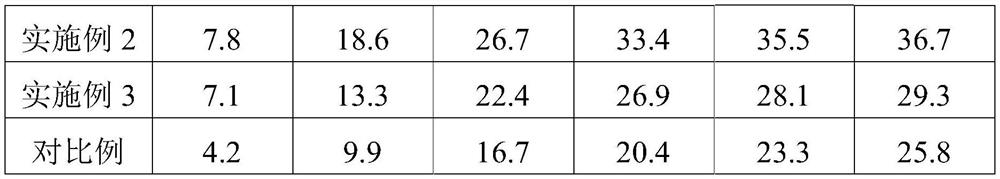
Image
Examples
Embodiment 1
[0022] prescription:
[0023] 138g of scutellaria, 104g of dog ridge, 104g of Shenjincao, 104g of Gentiana chinensis, 70g of Caulis Spatholobus, 104g of Fangji, 70g of geranium, 104g of mulberry branch, 52g of Wujiapi, 52g of Duhuo.
[0024] Preparation:
[0025] 1) The above medicinal materials were first distilled with water for 6 hours, and the volatile oil was collected for later use, and then continued to be decocted with water for 3 times, each time for 1 hour, and the two extracts were combined and concentrated into a thick paste with a relative density of 1.20 (cold measurement). To room temperature, add ethanol to make the alcohol content reach 40% (weight), after stirring evenly, let it settle down, get the supernatant and concentrate to a thick paste whose relative density is 1.35 (cold measurement).
[0026] 2) Prepare the gel matrix, the auxiliary materials are as follows:
[0027] Sodium polyacrylate 200g, polyvinylpyrrolidone 50g, crospovidone 50g, aluminum hy...
Embodiment 2
[0031] Prescription is identical with embodiment 1.
[0032] Preparation method:
[0033] 1) The above medicinal materials were first distilled with water for 8 hours, and the volatile oil was collected for later use, and then continued to be decocted and extracted twice, each time for 2 hours, and the two extracts were combined and concentrated into a thick paste with a relative density of 1.15 (cold measurement). To room temperature, add ethanol to make the alcohol content reach 70% (weight), after stirring evenly, let it settle down, get the supernatant and concentrate to a thick paste whose relative density is 1.35 (cold measurement).
[0034] 2) Prepare the gel matrix, the auxiliary materials are as follows:
[0035] Sodium polyacrylate 300g, polyvinylpyrrolidone 30g, crospovidone 30g, aluminum hydroxide 30g, citric acid 20g, glycerin 1000g, sorbitol 30g, propylene glycol 300g, laurocaprone 150g, edetate disodium 15g, ethylparaben 15g, menthol 30g, purified water 2500g....
Embodiment 3
[0039] Prescription is identical with embodiment 1.
[0040] Preparation method:
[0041] 1) Distill the above medicinal materials with water for 10 hours, collect the volatile oil for later use, then continue to add water to decoct and extract 4 times, the first time is 1.5 hours, the second time is 1 hour, the third time and the fourth time are 0.5 hours each, and combined twice The extract is also concentrated into a thick paste with a relative density of 1.10 (cold measurement), cooled to room temperature, added ethanol to make the alcohol content reach 55% (by weight), stirred well, left to settle, and the supernatant is concentrated to a relative density A thick paste of 1.35 (cold test).
[0042] 2) Prepare the gel matrix, the auxiliary materials are as follows:
[0043] Sodium polyacrylate 450g, polyvinylpyrrolidone 15g, crospovidone 15g, aluminum hydroxide 40g, citric acid 40g, glycerin 1200g, sorbitol 15g, propylene glycol 150g, laurocaprolactone 180g, edetate diso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com