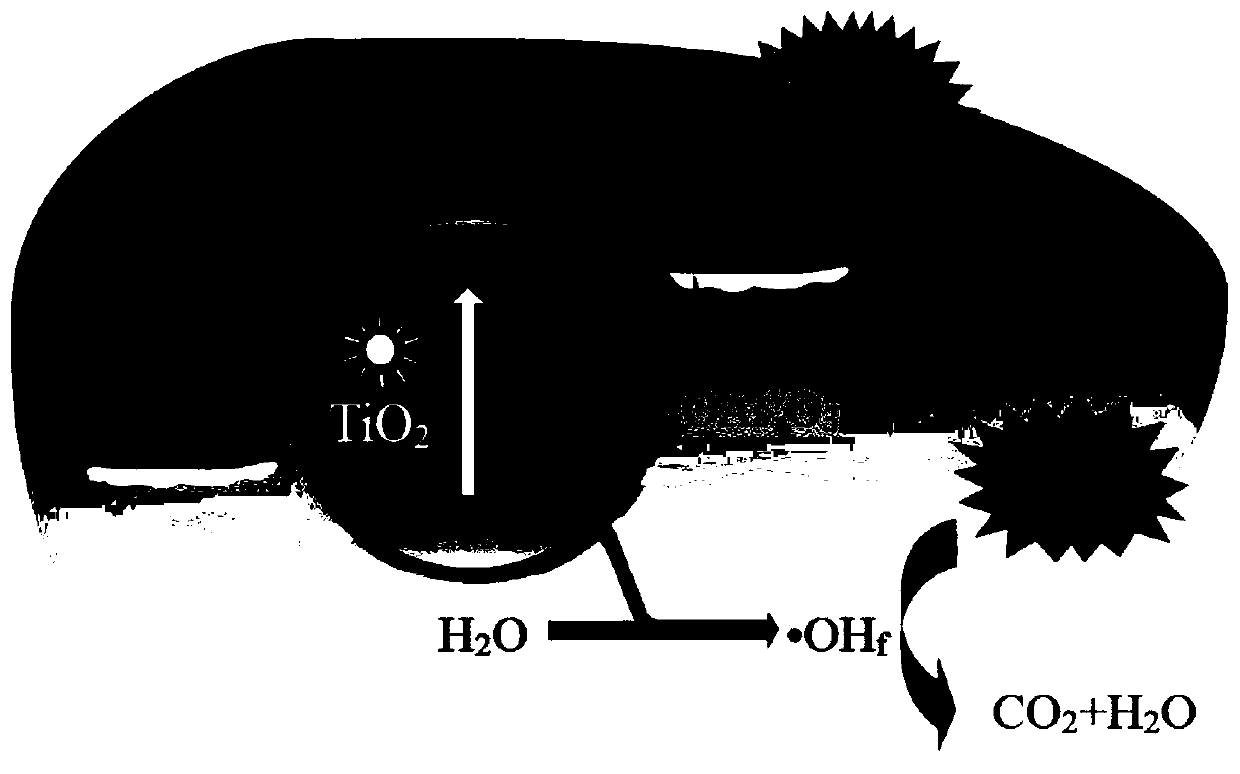
Method for promoting efficient photo-catalytic oxidation of phenolic pollutants by organic arsenic pollutants in situ
A technology for photocatalytic oxidation and pollutants, applied in water pollutants, chemical instruments and methods, oxidized water/sewage treatment, etc., can solve problems such as phenolic compound pollution, achieve efficient removal, easy preparation, and high performance effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1, crystal plane control TiO 2 Preparation of photocatalyst
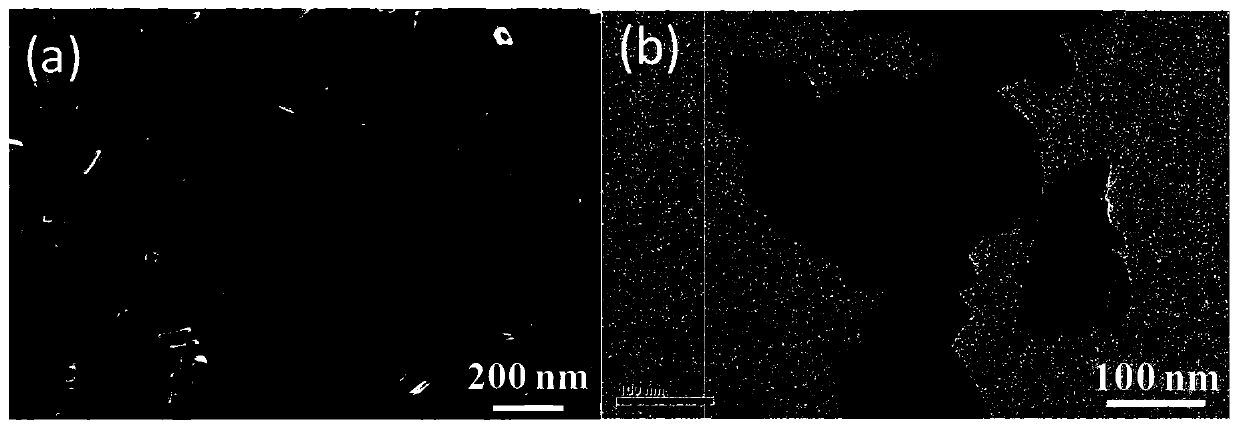
[0036] TiO used in this example 2 Photocatalyst regulation of TiO for crystal facets 2 The photocatalyst has a single-crystal crystallographic structure, is in the form of nano-crystal particles, has a regular quadrilateral sheet microscopic morphology, and has high-energy {001} polar crystal planes and low-energy {101} non-polar crystal planes exposed on the surface at the same time, and its preparation method As follows: add 20mL tetrabutyl titanate to 20mL HF aqueous solution with a concentration of 24wt.%, stir evenly to obtain a precursor solution; transfer the precursor solution to an autoclave, and react at a constant temperature of 170°C for 24h; cool down after the reaction After reaching room temperature, the obtained product was washed with ethanol, concentrated alkali and distilled water several times, dried at 60°C for 12h, and then calcined at 400°C for 5h in the air atmosphere to obta...
Embodiment 2
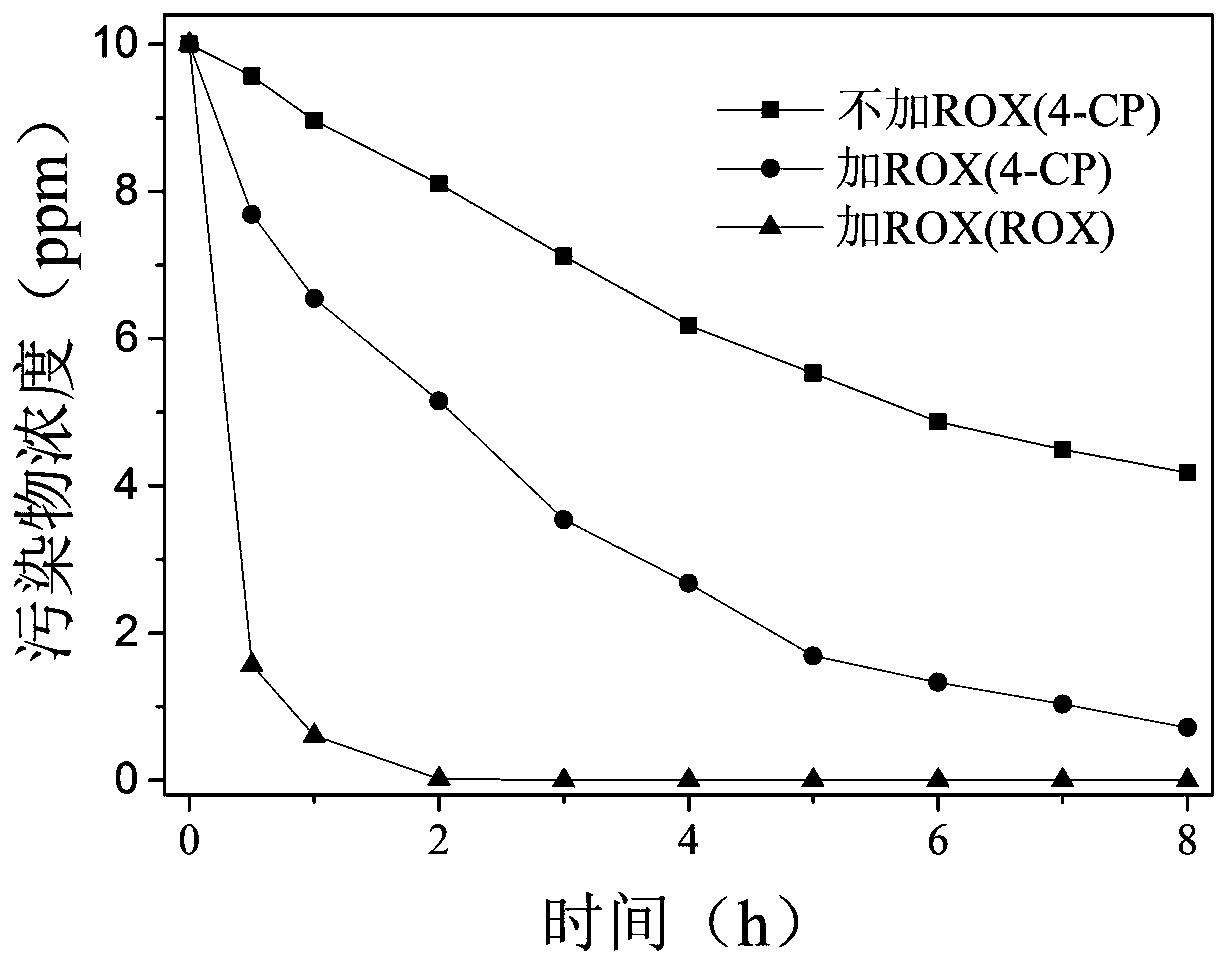
[0038] The influence of ROX in the synergistic degradation system of embodiment 2, chlorophenol (4-CP) and ROX
[0039] 1. In order to verify the influence of ROX in the synergistic degradation system of chlorophenol (4-CP) and ROX, three sets of experimental systems were set up:
[0040] First, at room temperature, in a 50mL beaker, the crystal facet-regulated TiO prepared in Example 1 was cleaned by ultrasonic cleaning. 2 Disperse in deionized water with a dispersion concentration of 0.7g / L;
[0041] Then: in the first group of experimental systems, add 4-CP to the beaker to a concentration of 10ppm; in the second group of experimental systems, add 4-CP and ROX stock solution to the beaker to both concentrations of 10ppm; the third group In the experimental system, add the ROX stock solution to the beaker to a concentration of 10ppm. The pH=6 of the three systems was adjusted by 0.1M HCl or NaOH.
[0042] Finally, the obtained systems were first magnetically stirred at 30...
Embodiment 3
[0046] Embodiment 3, the influence of pH p-chlorophenol (4-CP) and ROX synergistic degradation system
[0047] In order to verify the influence of pH on the synergistic degradation system of chlorophenol (4-CP) and ROX, the following experiments were set up:
[0048] First, at room temperature, in a 50mL beaker, the crystal facet-regulated TiO prepared in Example 1 was cleaned by ultrasonic cleaning. 2 Disperse in deionized water with a dispersion concentration of 0.7g / L; then add 4-CP and ROX stock solutions to the beaker until their concentrations are both 10ppm. Finally, the pH of the system was adjusted to 3, 4, 5, 6, 7, 8, and 9 by 0.1M HCl or NaOH, respectively. The resulting suspension was first magnetically stirred at 300 rpm for 30 minutes under light-proof conditions to fully carry out adsorption equilibrium on the catalyst surface, and then turn on ultraviolet light irradiation to carry out photocatalytic degradation reaction. The reaction time lasted for 8 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com