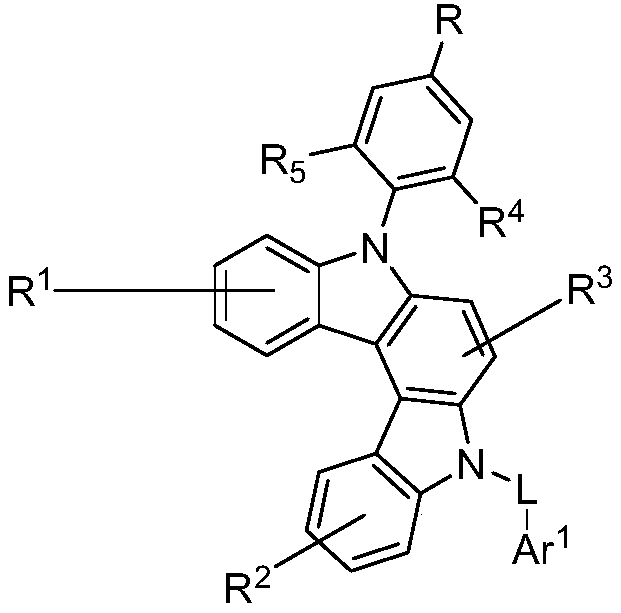
Organic compounds and application thereof
An organic compound, unsubstituted technology, applied in organic chemistry, chemical instruments and methods, luminescent materials, etc., can solve problems such as mismatch between electrons and holes, efficiency roll-off, shortened life, etc., to achieve improved life and good thermal stability High performance, key energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
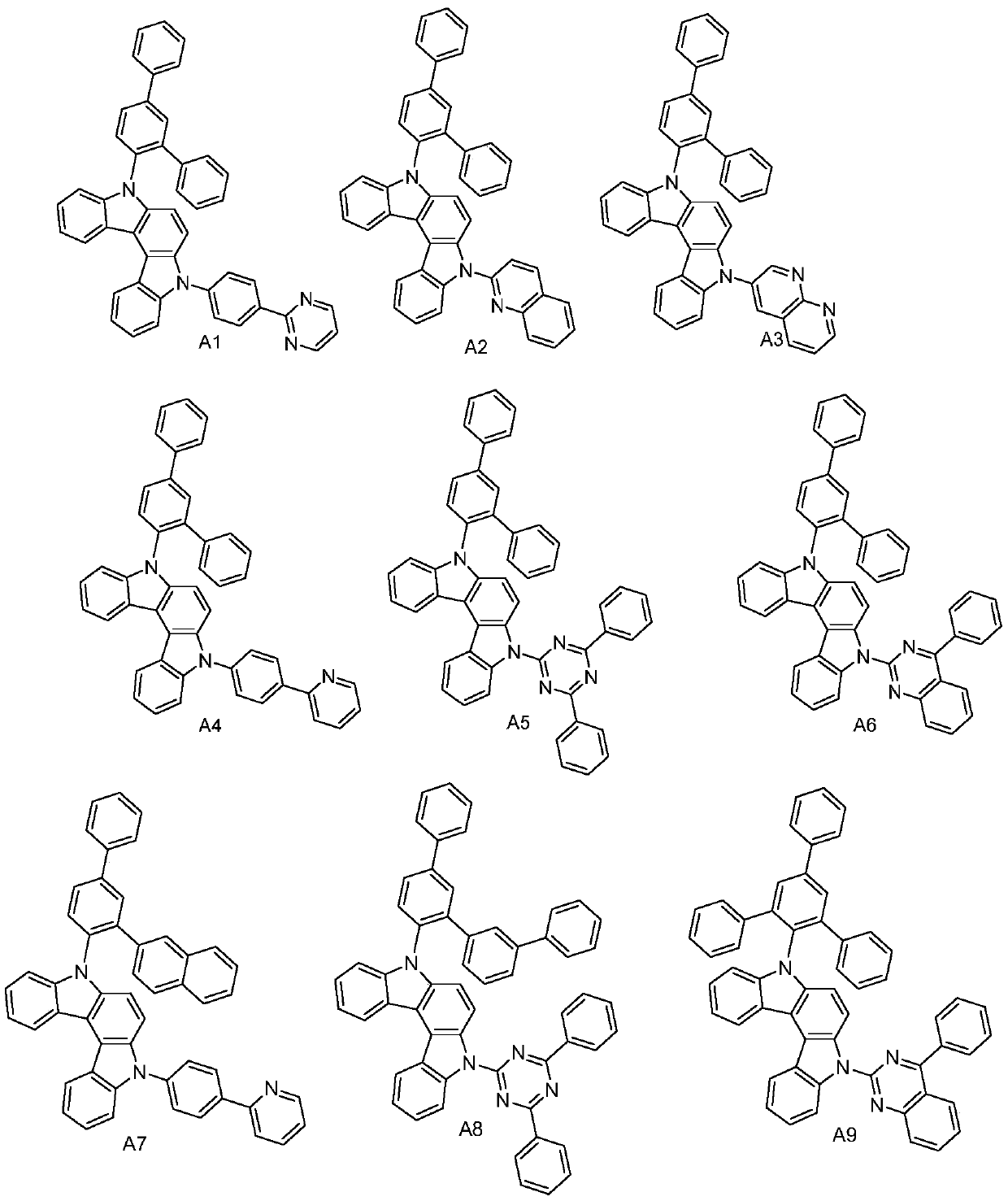
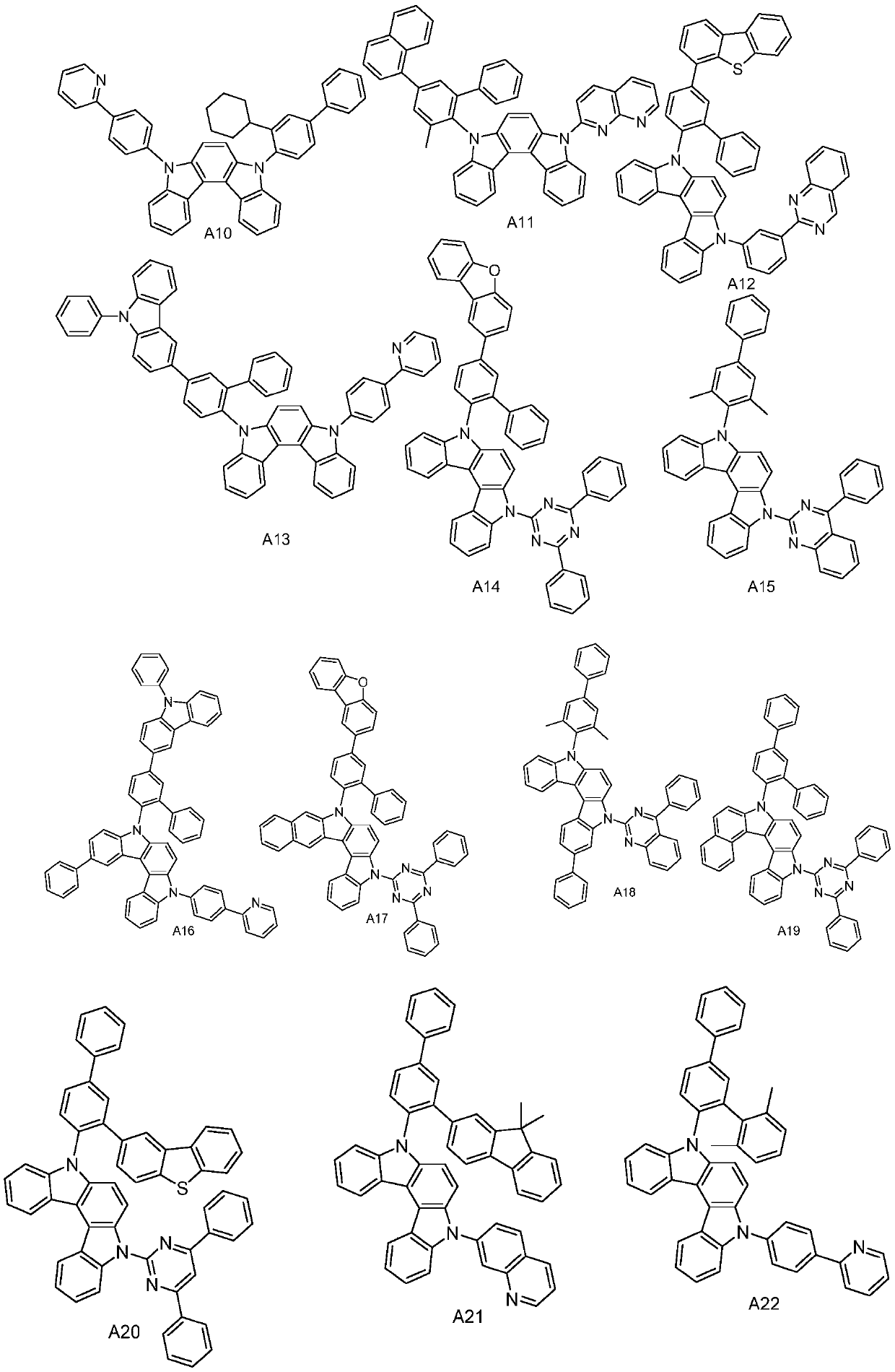
[0032] The synthesis of compound A1, the reaction equation is as follows:
[0033]
[0034] The synthesis method is as follows:
[0035] (1) In the reaction flask, add 25.6g (100mmol) 5,8-dihydroindolo(2,3-c)carbazole, 1,3-pyrimidine-5-(4-bromobenzene) 16.5g (100mmol ), Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), toluene 1500mL and sodium tert-butoxide 40g (300mmol), react at 100°C for 5h; stop the reaction after the reaction is completed, and cool the reactant to room temperature, add water, and extract with ethyl acetate, Concentration, organic phase column chromatography, the resulting solid was purified by recrystallization from toluene to obtain a white powder M1;
[0036] (2) Add 40.8g (100mmol) M1, 30.8g (100mmol) of 4-bromo-3-phenylbiphenyl, Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1000mL and sodium tert-butoxide 40g (300mmol), react at 140°C for 12h; stop the reaction after the reaction is complete, and cool the reactant to room temperature, add water, and extract...
Embodiment 2
[0039]The synthesis of compound A3, reaction equation is as follows:
[0040]
[0041] The synthesis method is as follows:
[0042] (1) In the reaction flask, add 25.6g (100mmol) 5,8-dihydroindolo(2,3-c)carbazole, 3-bromonaphthyridine (100mmol), Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), toluene 1500mL and sodium tert-butoxide 40g (300mmol), react at 100°C for 5h; stop the reaction after the reaction is completed, and cool the reactant to room temperature, add water, and extract with ethyl acetate, Concentration, organic phase column chromatography, the resulting solid was purified by recrystallization from toluene to obtain a white powder M1;
[0043] (2) Add 40.8g (100mmol) M1, 30.8g (100mmol) of 4-bromo-3-phenylbiphenyl, Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1000mL and sodium tert-butoxide 40g (300mmol), react at 140°C for 12h; stop the reaction after the reaction is complete, and cool the reactant to room temperature, add water, and extract with ethyl acetate , con...
Embodiment 3
[0046] The synthesis of compound A8, reaction equation is as follows:
[0047]
[0048] The synthesis method is as follows:
[0049] (1) In the reaction flask, add 25.6g (100mmol) of 5,8-dihydroindolo(2,3-c)carbazole, 38g (100mmol) of 4-bromo-(3-biphenyl)biphenyl, PD 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1000mL and sodium tert-butoxide 40g (300mmol), react at 140°C for 5h; stop the reaction after the reaction is completed, and cool the reactant to room temperature, add water, and extract with ethyl acetate , concentrated, organic phase column chromatography, and the obtained solid was purified by recrystallization in toluene to obtain a white powder M1;
[0050] (2) Add M1 (100mmol), 2-chloro-4,6-diphenyltriazine (100mmol), potassium carbonate 40g (300mmol) and DMF 500mL to the reaction flask and react at 140°C for 5h; stop the reaction after the reaction is completed , and the reactant was cooled to room temperature, water was added, a solid was precipitated, filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com