Application of merocyanine-coumarin fluorophore to detection of hydrogen sulfide in mitochondria
A technology of coumarin and hydrogen sulfide, applied in fluorescence/phosphorescence, organic chemistry, luminescent materials, etc., can solve the problems of inability to meet actual needs, long response time, etc., and achieve the effect of rapid response, high sensitivity and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of coumarin aldehyde derivative
[0029] Preparation of Compound 2: Dissolve 3.88g of 4-N,N diethyl salicylaldehyde, 6.0mL of diethyl malonate and 2mL of hexahydropyridine in 65mL of absolute ethanol, and heat to reflux for 6h. Spin to dry the solvent, add 40 mL of concentrated hydrochloric acid and 40 mL of glacial acetic acid into the eggplant-shaped bottle, and reflux for six hours. After cooling to room temperature, the reaction solution was poured into 200 mL of ice water, and 40% NaOH was added dropwise with stirring in an ice bath until pH ≈ 5, and a large amount of yellow precipitate was obtained. Suction filtration, washing the filter cake with a large amount of ice water. Drying in vacuo yielded 3.66 g of product, yield 83.9%.
[0030] Synthesis of Compound Co: N at 30°C 2 Under protection, slowly drop 6mL of anhydrous DMF into 6mL of POCl 3 After stirring for 30 min, a reddish-brown solution was obtained. 4.56g of compound 2...
Embodiment 2
[0031] Embodiment 2: Preparation of CMC series probes
[0032] Indole salt synthesized according to literature (Pardal A.C., Ramos S.S., Santos P.F., Reis L.V., Almeida P., Synthesis and Spectroscopic Characterization of N-Alkyl Quaternary AmmoniumSalts Typical Precursors of Cyanines, Molecules, 2002, 3:320-330) Derivative (MR) 1.0mmol and coumarin aldehyde derivative 1.0mmol were dissolved in 20mL ethanol solution. Heated to reflux for 12h, and the solvent was spin-dried under reduced pressure after the reaction was completed. The resulting residue was separated by column chromatography (CH 2 Cl 2 :CH 3 OH, 20:1 to 10:1), to obtain the corresponding CMC series products.
[0033] CM-NC 1 , Yield: 56%. 1 H NMR (300MHz, Chloroform-d) δ10.05(s, 1H), 8.59(d, J=15.9 Hz, 1H), 8.11(d, J=9.1Hz, 1H), 8.01(d, J=15.9Hz ,1H),7.69–7.37(m,4H),6.71(dd,J=9.1, 2.4Hz,1H),6.47(d,J=2.2Hz,1H),4.31(s,3H),3.52(q, J=7.1Hz,4H),1.84(s,7H),1.29(t,J=7.1Hz,6H). 13 C NMR(75MHz,Chloroform-d)δ181.17...
Embodiment 3
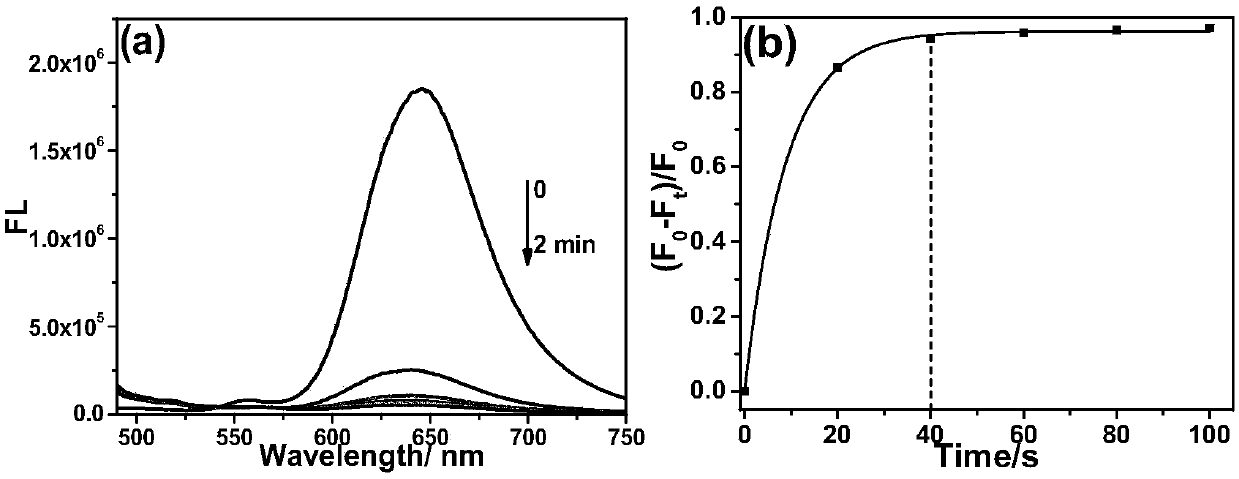
[0035] Example 3: Probe CM-NC 6 Fluorescence Spectrum of Response Speed to Hydrogen Sulfide
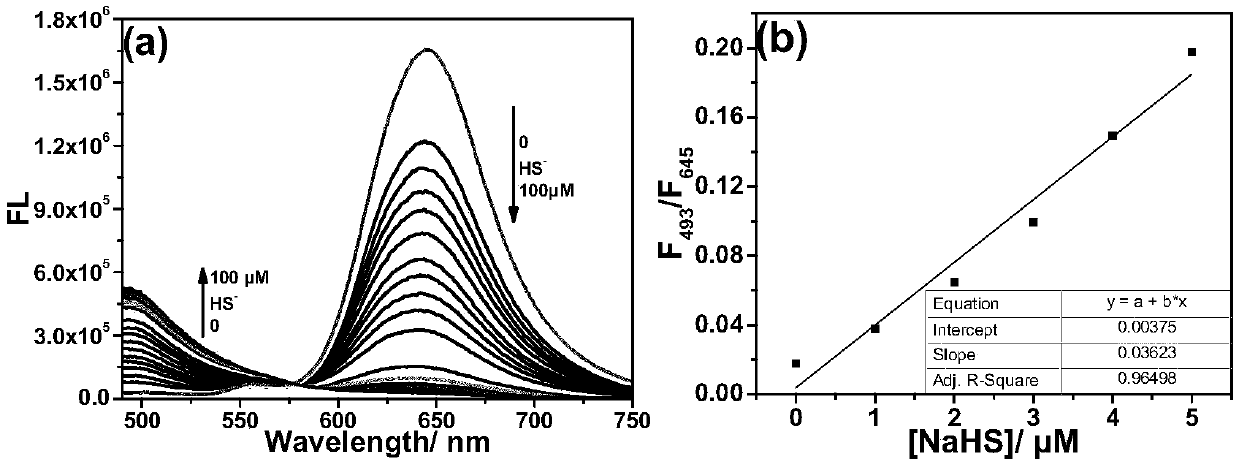
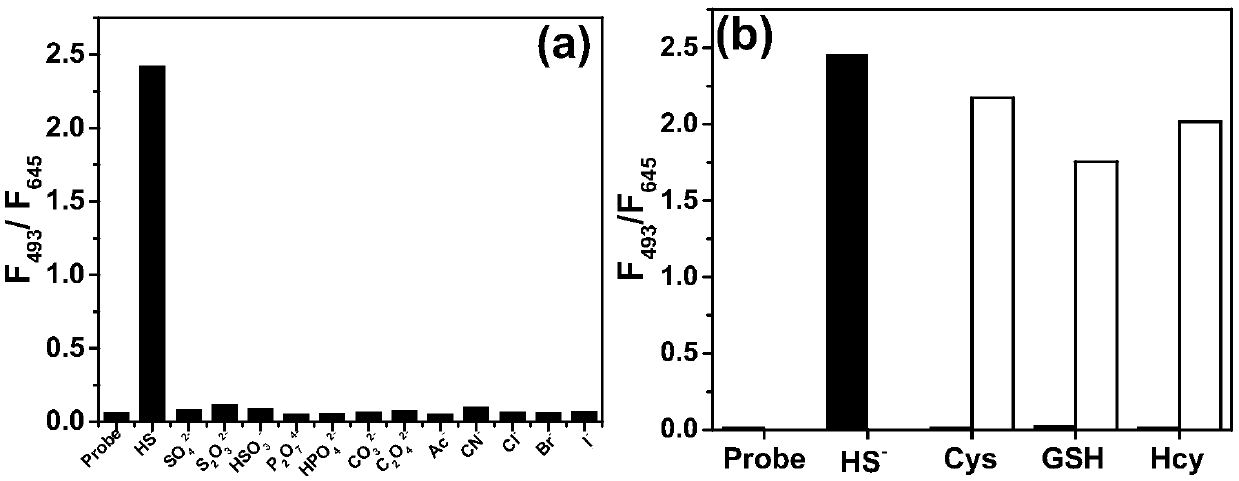
[0036] The test concentration of the fluorescence spectrum used in the present invention is 10 μM, the solvent is 20 mM PBS solution with pH=7.4 mixed with 2% DMSO, and when the emission spectrum is measured, the excitation wavelength is 475 nm. NaHS was used as the hydrogen sulfide donor. Add the fluorescent probe to the PBS solution, add 20 equivalents of NaHS and mix well, then perform the corresponding fluorescence spectrum test at regular intervals. Such as figure 1 As shown, to 10μM CM-NC 6 When 200 μM NaHS was added to the PBS solution, the emission peak of the merocyanine / coumarin hybrid fluorophore at 650 nm decreased significantly with time, and nearly disappeared after 2 min. Through the fluorescence change rate curve (F 0 -F t ) / F 0 It can be seen that the reaction is close to equilibrium when the reaction reaches 40s, and the reaction degree reaches more than 95...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com