Preparation method of lactone compound
A technology of compounds and cyclic compounds, applied in the field of preparation of lactone compounds, can solve the problems of low yield, achieve simple process, reduce hydrolysis, improve selectivity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
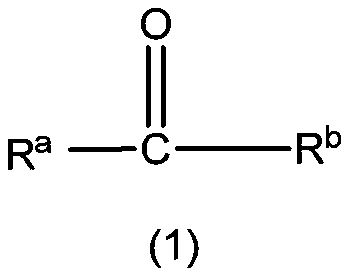
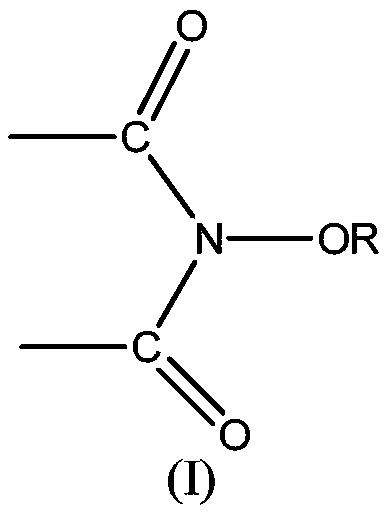
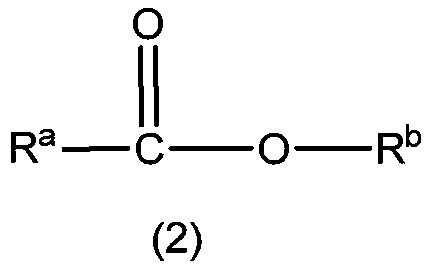
[0064] In the preparation method of the first lactone compound, alcohol can be the secondary alcohol represented by formula (3), R c , R d The expression "an organic group having a carbon atom at a bonding site to an adjacent hydroxyl carbon atom" includes hydrocarbon groups and heterocyclic groups. The hydrocarbon group may be a straight chain or branched chain substituted hydrocarbon group of various carbon chain lengths, and preferably includes aliphatic groups with about 1 to 20 carbon atoms (preferably 1 to 15 carbon atoms, more preferably 1 to 10 carbon atoms). Hydrocarbyl (including alkyl, alkenyl or alkynyl), aliphatic hydrocarbyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cyclooctyl, cyclododeca Alicyclic hydrocarbon group (cycloalkyl or cycloalkenyl) with 3 to 20 members (preferably 3 to 15 members, more preferably 5 to 8 members), such as alkyl, phenyl, naphthyl, anthracenyl, phenanthryl, fluorene An aromatic hydrocarbon group ...
Embodiment 1
[0119] In connection with O 2 Into a 25ml three-necked bottle of balloon, add 2mmol of cyclohexanol, 4g of 1,4-dioxane, 0.4mmol of NHPI, 0.2mmol of AIBN, 4mmol of cyclohexanone, and the mass percentage of Sn is 3.5wt%Sn- 68mg of beta molecular sieve (the mole percentage of Sn is about 0.63 mol% of cyclohexanol), stirred and reacted for 12 hours, and the reaction temperature was controlled to be 75°C. After the reaction, the reaction solution was cooled to room temperature, and samples were taken for detection by GC. The results are shown in Table 1.
Embodiment 2
[0121] In connection with O 2 In a 25ml three-neck bottle of balloon, add 2mmol of cyclohexanol, 4g of 1,4-dioxane, 0.4mmol of NHS, 0.2mmol of AIBN, 4mmol of cyclohexanone, and the mass percentage of Sn is 3.5wt%Sn- 68mg of beta molecular sieve (the mole percentage of Sn is about 0.63 mol% of cyclohexanol), stirred and reacted for 12 hours, and the reaction temperature was controlled to be 75°C. After the reaction, the reaction solution was cooled to room temperature, and samples were taken for detection by GC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com