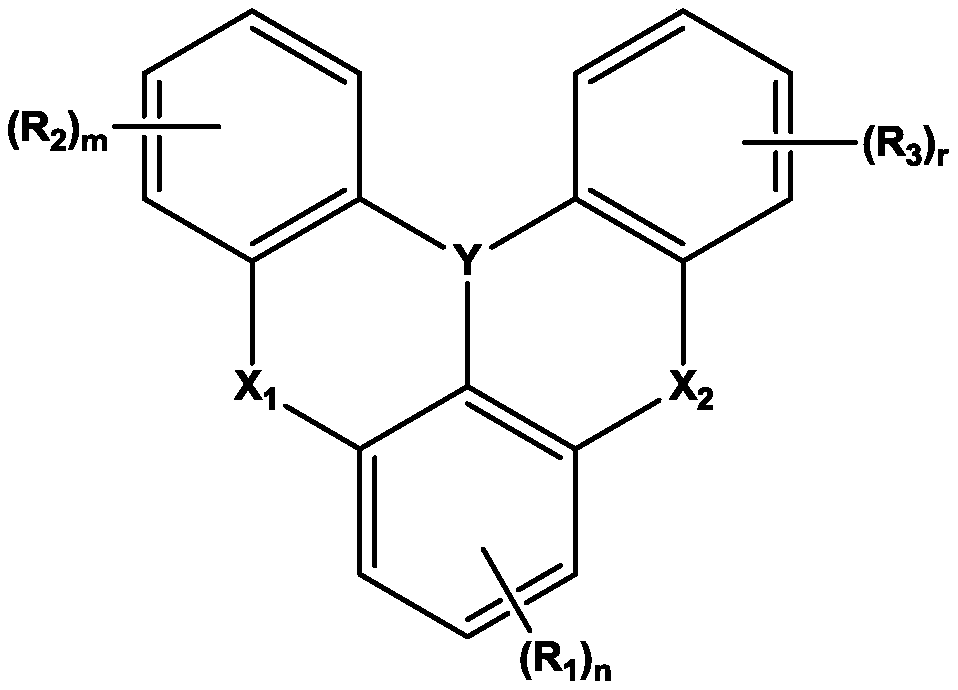
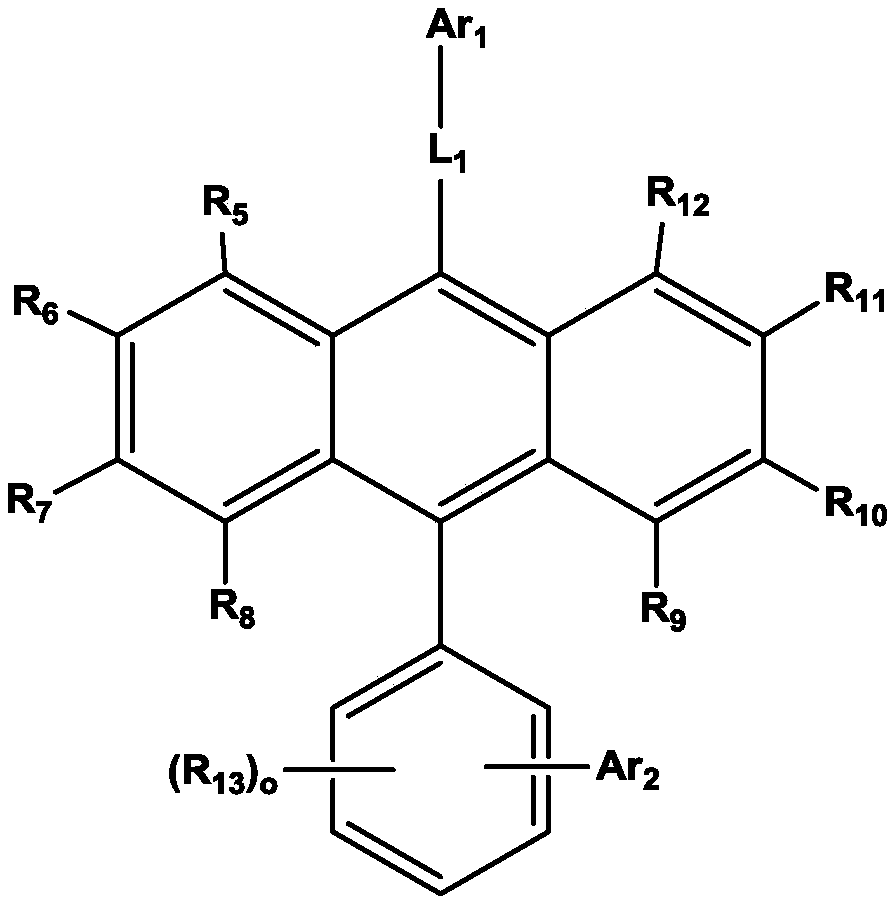
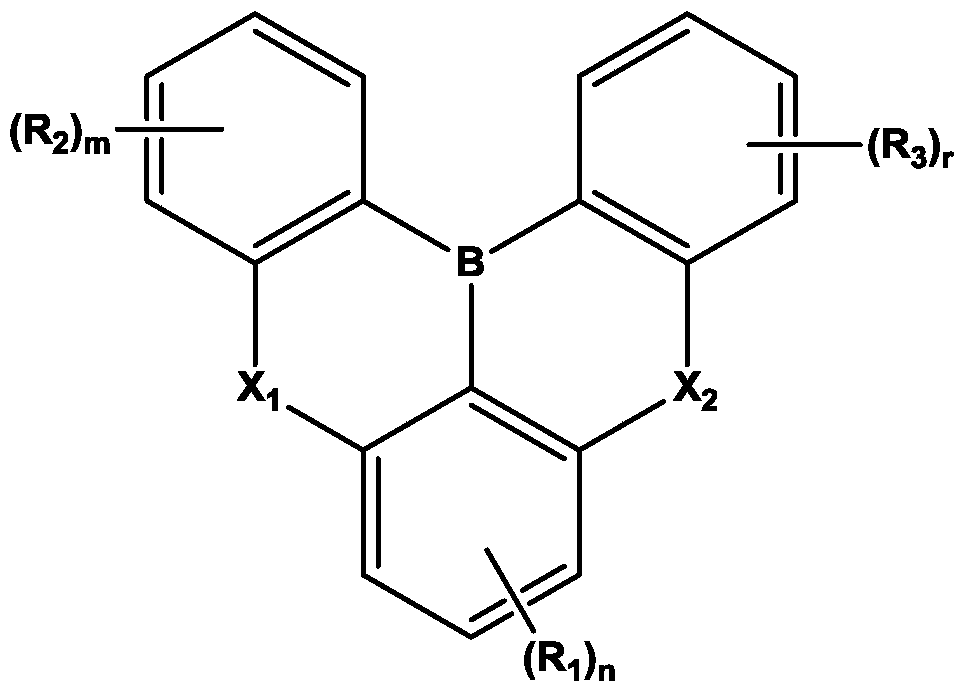
Organic electroluminescent device
An electroluminescent element and luminescent technology, which is applied in the direction of electrical components, electric solid-state devices, circuits, etc., can solve the problems of difficult to achieve dark blue, short life, optical loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1-1
[0143]
[0144] 8.9g (20mmol) of the starting material 1 was dissolved in butylbenzene (250ml) and then cooled to 0°C. Under a nitrogen atmosphere, 24.7 ml (42 mmol) of a 1.7 M butyllithium solution (in Pentane) was added, and the mixture was stirred at 60°C for 2 hours.
[0145] After that, the reactant was cooled to 0°C again, 4.0 ml (42 mmol) of BBr3 was added, and the mixture was stirred at room temperature for 0.5 hours. The reactant was cooled to 0°C again, and 7.3 ml (42 mmol) of N,N-diisopropylethylamine was added, followed by stirring at 60°C for 2 hours.
[0146] The reaction liquid was cooled to room temperature, and the organic layer was extracted with ethyl acetate (Ethyl acetate) and water (Water). After removing the solvent of the extracted organic layer, it was purified by silica gel column chromatography (DCM / Hexane). Then, it was recrystallized and refined using a DCM / acetone (Acetone) mixed solvent to obtain 1.7 g of the above-mentioned compound 1-1 with a yiel...
Synthetic example 1-2
[0149]
[0150] Except that 9.9 g (20 mmol) of the starting material 1-3 was used instead of the starting material 1-1, the experiment was performed in the same manner as in Synthesis Example 1-1 to obtain 2.16 g of the above compound 1- with a yield of 23.0% 3.
[0151] MS(MALDI-TOF)m / z: 470[M]+
Synthetic example 1-3
[0153]
[0154] Except that 10.6 g (20 mmol) of the starting material 1-5 was used instead of the starting material 1-1, the experiment was performed in the same manner as in Synthesis Example 1-1 to obtain 2.3 g of the above compound 1- with a yield of 23.2% 5.
[0155] MS(MALDI-TOF)m / z: 502[M]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com