Self-cross-linking waterborne light-curing polyurethane, preparation method and applications thereof
A photocuring and self-crosslinking technology, applied in the field of photocuring, can solve the problems of limited photoinitiation efficiency, limited degree of crosslinking and curing, and few double bond molecules, and achieve enhanced initiation efficiency, fast curing speed and high photoinitiation efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
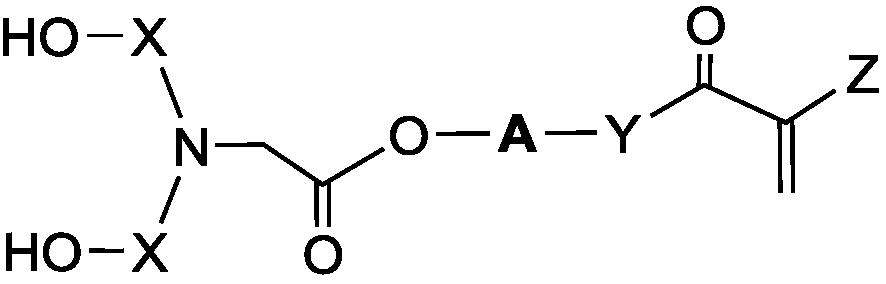
[0057] Further, the present invention provides a method for preparing the above-mentioned unsaturated polyol, which includes the following steps: 1) mixing hydroxyalkyl (meth)acrylate or hydroxyalkyl (meth)acrylamide and halogen Acetyl halide reaction synthesis to obtain an intermediate product containing halogen and unsaturated bonds; 2) reacting the intermediate product with an amino compound to obtain the unsaturated polyol.
[0058] Preferably, step 1) dissolve the hydroxyalkyl (meth)acrylate or hydroxyalkyl (meth)acrylamide in a solvent, add haloacetyl halide dropwise at low temperature, and react under the action of a catalyst to obtain an intermediate product. The hydroxyalkyl (meth)acrylate is selected from one or more of hydroxyethyl (meth)acrylate and hydroxypropyl (meth)acrylate; the hydroxyalkyl (meth)acrylamide is selected from One or more of N-methylolacrylamide, N-hydroxyethylacrylamide, N-hydroxypropylacrylamide; the haloacetyl halide is selected from any one o...
Embodiment
[0103] The following examples are given to illustrate the present invention, and those skilled in the art can understand that the examples are only exemplary descriptions, not exhaustive descriptions. The preparation examples are compound synthesis examples, and the chemical raw materials and reagents involved are all commercially available or synthesized according to published documents.
[0104] Measure the flexibility of the coating film according to the standard GB / T 1731-93: After the paint film is completely cured, use both hands to press the sample of the coating film tightly on the shaft rod with a standard diameter, and bend it around the rod; the paint film is used after bending. 4 times magnifying glass observation.
[0105] Adhesion test: When measuring, make the tip of the tester needle touch the coating film, shake the handle clockwise evenly, the speed is 80 rpm, and the scratch length is 7.5±0.5mm. Scratches were inspected and graded with a 4x loupe.
[0106]...
preparation example 1
[0109] Preparation Example 1: Synthesis of Unsaturated Polyols
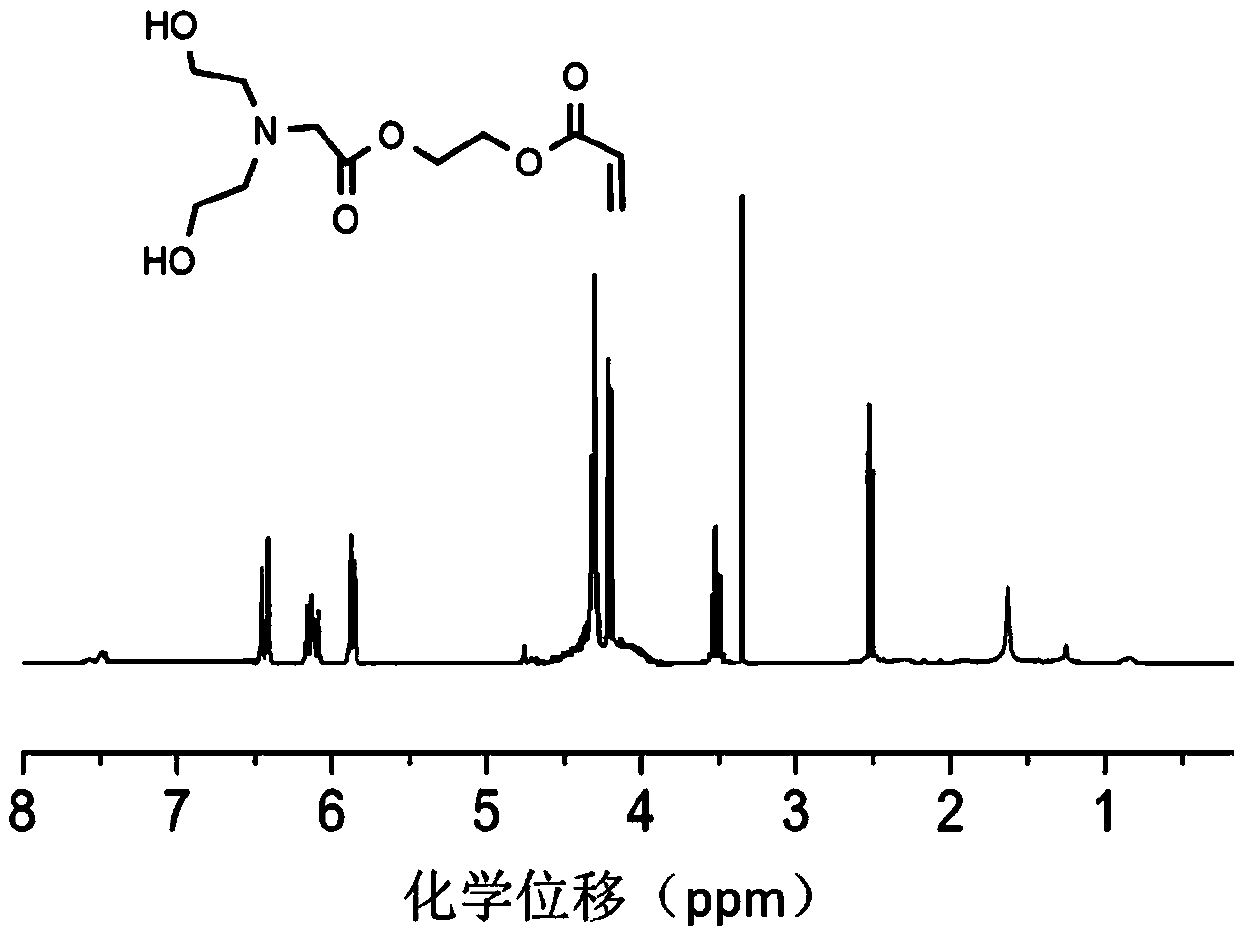
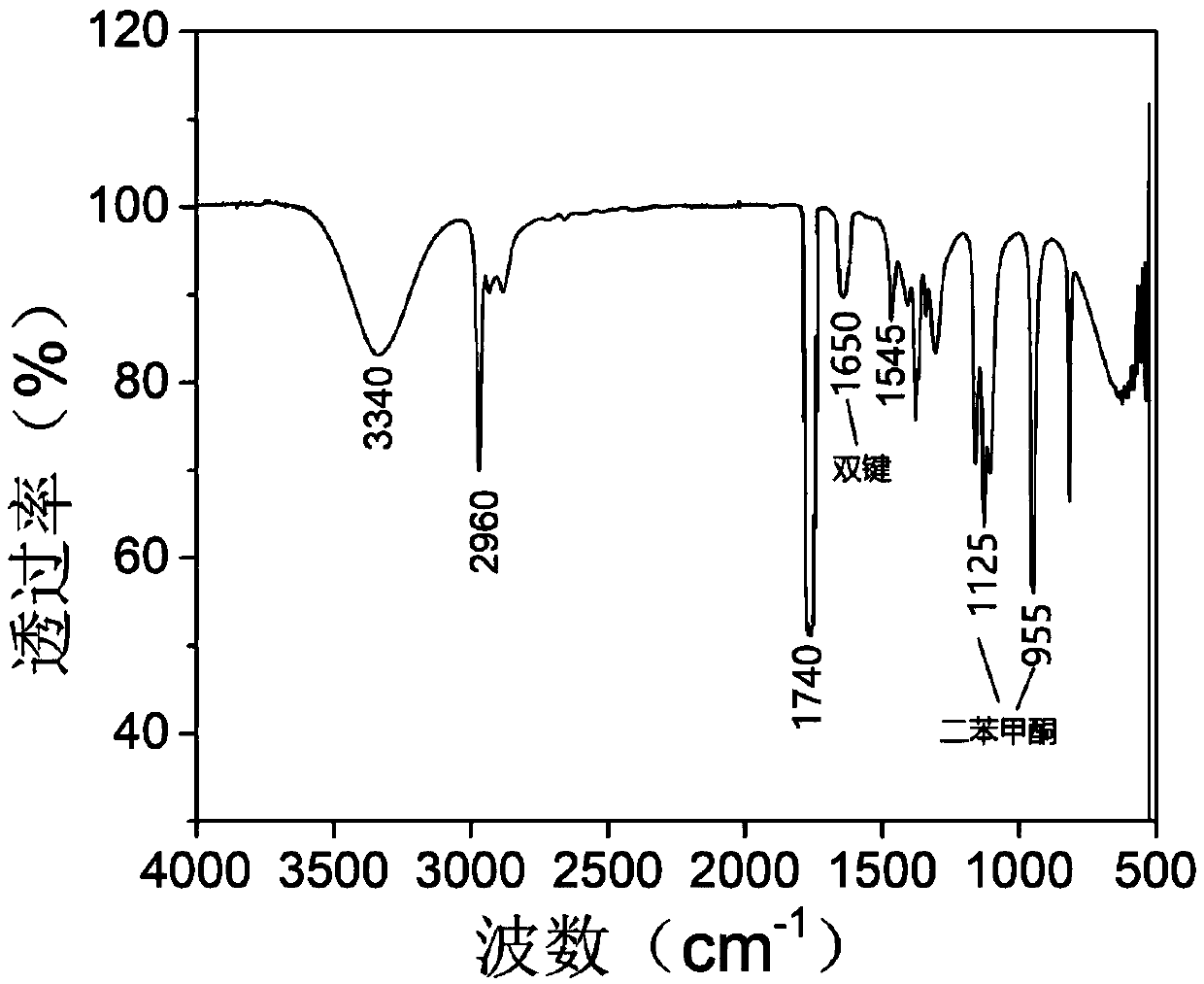
[0110] Hydroxyethyl acrylate (11.5 mL, 0.1 mol) was dissolved in dichloromethane, and bromoacetyl bromide (9.6 mL, 0.11 mol) was added dropwise to the solution at low temperature. The molar ratio of bromoacetyl bromide to hydroxy ethyl acrylate was 1.1:1, reacted under the action of sodium hydroxide (4g, 0.1mol) for 0.5h, raised the temperature to 30°C, and continued to react for 16h. After purification and drying, 2-(2-bromoacetoxy)ethyl acrylate ( 16.5 g, the yield was 70%); after that, 2-(2-bromoacetoxy) ethyl acrylate (11.8 g, 0.05 mol) and diethanolamine (6.3 g, 0.06 mol) were dissolved in dioxane , under the catalysis of triethylamine (6.1g, 0.06mol), reacted at 60°C for 5h, separated and purified, and dried to obtain 2-(N,N-dihydroxyethyl)-2-acetoxyethyl acrylate (10.2 g, 78% yield). For the NMR spectrum of the product, see figure 1 , 1 H NMR(δ / ppm):2.51(m,4H,CH 2 ),3.35(s,2H,CH 2 ),3.52(m,4H,CH 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com