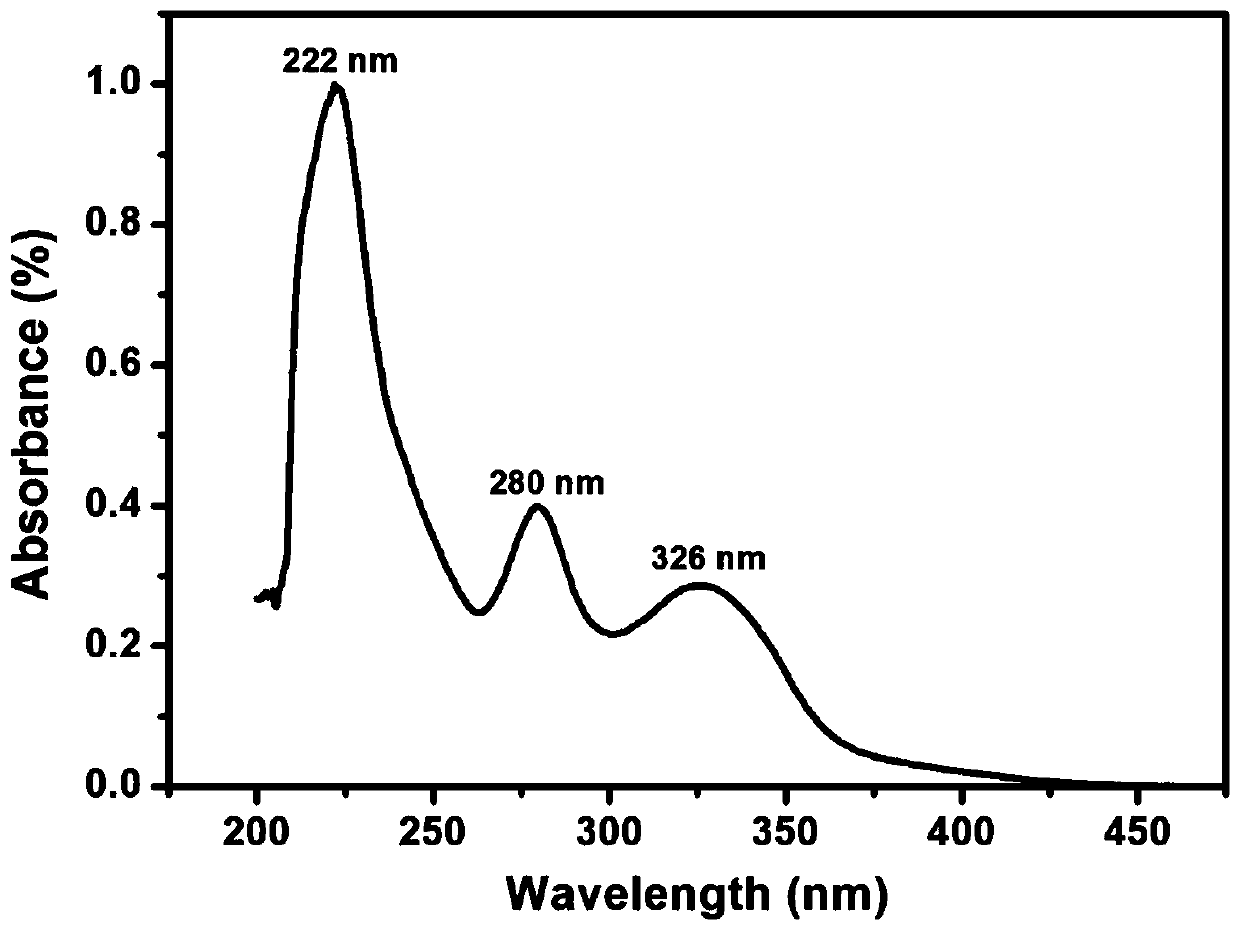
Aromatic diamine monomer and preparation method thereof
A technology of diamine monomer and aromatic type, which is applied in the field of aromatic diamine monomer and its preparation, can solve the problems of process controllability, poor repeatability, large specific surface area of nanoparticles, and volatilization loss, etc., and achieve excellent resistance Ultraviolet radiation, high process controllability, and the effect of delaying damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a method for preparing the aromatic diamine monomer described in the above technical solution, comprising the following steps:
[0049] a) carry out etherification reaction with the compound of structure shown in formula (I) with methoxyphenol in the presence of basic catalyst, obtain the compound with structure shown in formula (II); Described methoxyphenol is 3-methyl oxyphenol, 4-methoxyphenol or 3,5-dimethoxyphenol;
[0050] X-R 1 Formula (I);
[0051] In formula (I), X is fluorine, chlorine, bromine, iodine, methanesulfonyloxy, trifluoromethanesulfonyloxy or p-toluenesulfonyloxy;
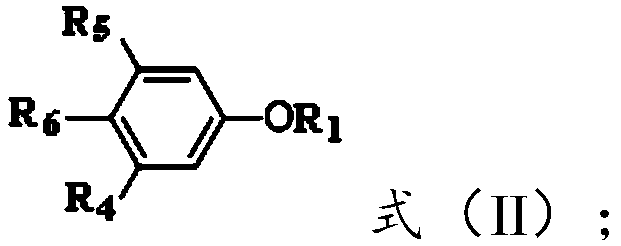
[0052]
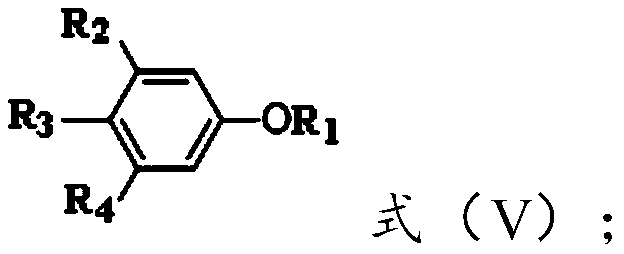
[0053] In formula (II), R 5 and R 6 independently selected from hydrogen or and R 5 with R 6 different;
[0054] b) performing a Friedel-Crafts acylation reaction on a compound having a structure shown in formula (II) and a dinitrobenzoyl halide in the presence of a catalyst to obtain a compound having a structure shown in formula...
Embodiment 1
[0130] (1) 37.24g (0.30 mole) 3-methoxyphenol, 54.47g (0.33 mole) n-hexane bromide, 49.76g (0.36 mole) potassium carbonate, 2.49g (0.015 mole) potassium iodide and 275g acetonitrile were added to In the reaction flask, the reaction system was then heated to 80°C for 6 hours. After being cooled to room temperature, it was added to 1500 milliliters of water, the product was extracted with dichloromethane, dried over anhydrous magnesium sulfate, the solvent was concentrated, and purified to obtain 53.74g of the representative compound 1-hexyloxy-3-methoxy with the structure shown in formula (II) The refined product of phenylbenzene, the yield is 86.0%.
[0131] 1H NMR (400MHz, DMSO) δ = 7.152 (t, J = 8.0Hz, 1H), 6.445-6.510 (m, 3H), 3.920 (t, J = 6.4Hz, 2H), 3.718 (s, 3H), 1.735 -1.625 (m, 2H), 1.460–1.355 (m, 2H), 1.345–1.205 (m, 4H), 0.875 (t, J=6.8Hz, 3H).
[0132] (2) 27.67g (0.12 moles) of 3,5-dinitrobenzoyl chloride, 17.33g (0.13 moles) of aluminum trichloride, 350g of 1...
Embodiment 2
[0139] (1) 62.07g (0.50 mole) 3-methoxyphenol, 78.75g (0.45 mole) p-bromofluorobenzene, 4.76g (0.025 mole) cuprous iodide, 82.93g (0.60 mole) potassium carbonate and 420gN, N-dimethylformamide was sequentially added into the reaction flask, and the reaction system was heated to 150° C. for 8 hours under the protection of nitrogen. After being cooled to room temperature, it was added to 2500 ml of water, the product was extracted with dichloromethane, washed with dilute hydrochloric acid, dried over anhydrous magnesium sulfate, the solvent was concentrated, and 77.58 g of the representative compound 1-(4-fluoro The refined product of phenoxy)-3-methoxybenzene, the yield is 79.0%.
[0140] 1 H NMR (400MHz, DMSO) δ=7.295–7.175(m,3H),7.105–7.025(m,2H),6.703(dd,J=8.4Hz,2.2Hz,1H),6.557(t,J=2.2Hz , 1H), 6.504 (dd, J = 8.2Hz, 2.2Hz, 1H), 3.728 (s, 3H).
[0141] (2) 27.67g (0.12 mol) 3,5-dinitrobenzoyl chloride, 17.33g (0.13 mol) aluminum trichloride, 360g1,2-dichloroethane and 21.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com