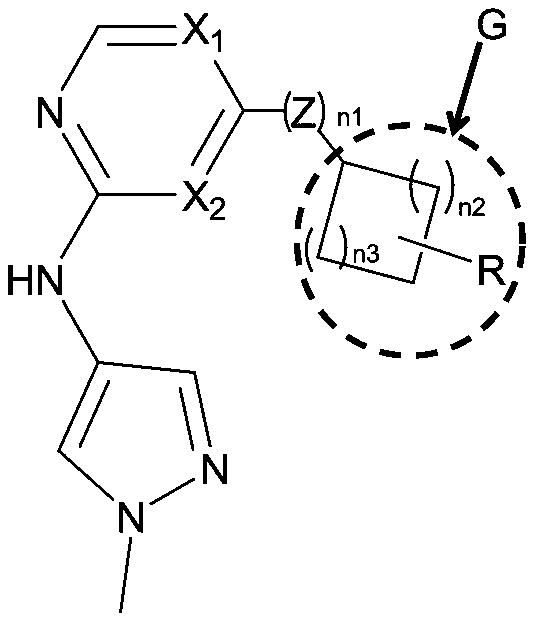
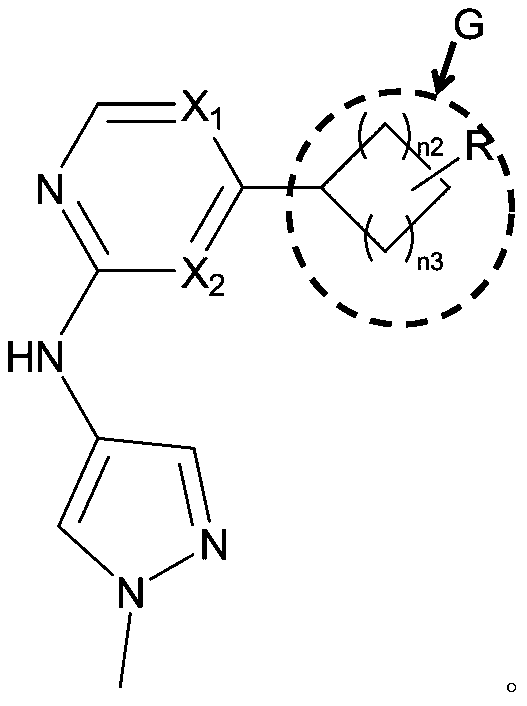
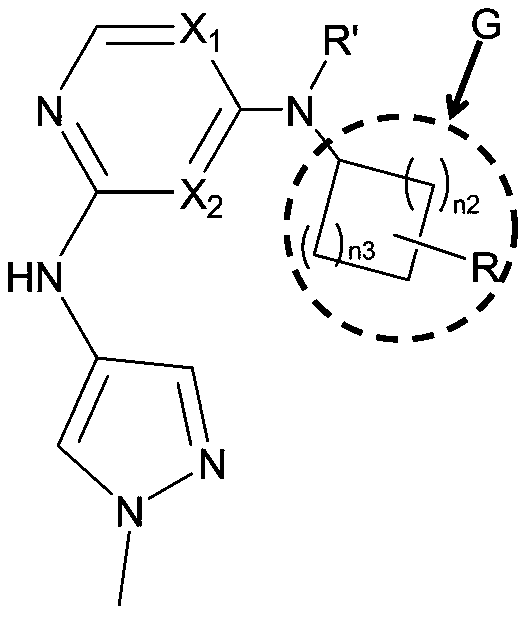
Small molecule compound
A technology of small molecule compounds and compounds, applied in digestive system, organic chemistry, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1, the reaction equation of synthetic compound TDM-180656 is as follows:
[0096]
[0097] Step 1: Example 56c
[0098] Compound 56a (1.2g, 8.0mmol), compound 56b (2.97g, 9.6mmol), Pd(dppf)Cl 2 (585mg, 0.8mmol), sodium carbonate (2.0g, 19.2mmol), 1,4-dioxane (30mL) and water (5mL) were added into a three-neck flask. Under the condition of the system water pump, the nitrogen was pumped and replaced three times. The reaction solution was reacted at 90° C. for 2 hours and then cooled. After the reaction was completed, the crude product was concentrated under reduced pressure and purified by column chromatography (eluent: petroleum ether / EtOAc=1 / 1) to obtain a colorless oily liquid compound 56c, namely compound 4-(2-chloropyrimidin-4-yl )-tert-butyl 5,6-dihydropyridine-1(2H)-carboxylate (1.9 g, 80% yield).
[0099] LCMS[M-55] + =240.0
[0100] 1 H NMR (400MHz, Chloroform-d) δ8.57 (d, J = 5.2Hz, 1H), 7.26 (d, J = 5.2Hz, 1H), 7.81 (s, 1H), 7.02 (s, 1H), 4...
Embodiment 2
[0119] Embodiment 2: The reaction equation of synthetic compound TDM-180658 is as follows:
[0120]
[0121] Add compound 56g (80mg, 0.31mmol), triethylamine (313mg, 3.1mmol) and N,N-dimethylformamide (5mL) into the reaction flask, and stir for about 6 minutes. Compound 58a, propionic acid (46 mg, 0.62 mmol) and HATU (177 mg, 0.47 mmol) were then added. The mixture was stirred at room temperature for 5 hours. After the reaction was completed, it was poured into water (2 mL), extracted with ethyl acetate (20 mL x 3). The organic layers were combined, washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The crude product was purified by column chromatography (eluent: methanol / ethyl acetate=1 / 20) to obtain yellow solid compound 58, namely 1-( 4-(2-((1-Methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)piperidin-1-yl)propan-1-one (73.4 mg, 34% yield) .
[0122] LCMS[M+1] + =315.1
[0123] 1 H NMR (400MHz, Chloroform-d) δ8.31(d, J=5...
Embodiment 3
[0131] Embodiment 3, the general synthesis method of synthetic compound TDM-180662
[0132]
[0133] Step 1: Example 62b
[0134] Using compound 56g as the starting material, the synthesis steps are similar to those of compound 58. Compound 62b was obtained as light yellow solid compound 2-(4-(2-((1-methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)piperidin-1-yl)-2- Oxoethyl acetate (90 mg, 32% yield).
[0135] LCMS[M+1] + =359.1
[0136] 1 H NMR (400MHz, Chloroform-d) δ8.32(d,J=4.8Hz,1H),7.82(s,1H),7.51(s,1H),6.89(s,1H),6.54(d,J= 4.8Hz,1H),4.65-4.85(m,3H),3.92(s,3H),3.74-3.85(m,1H),3.13-3.27(m,1H),2.70-2.86(m,2H),2.22 (s,3H),1.94-2.06(m,2H).
[0137] Step 2: Example 62 (TDM-180662)
[0138] To a solution of compound 62b (90 mg, 0.25 mmol) in tetrahydrofuran (5 mL) was added aqueous lithium hydroxide (1.5 mL, 1M). The mixture was stirred at room temperature for 3 hours. The reaction solution was diluted with water and extracted with ethyl acetate (15 mL*7). The organi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com