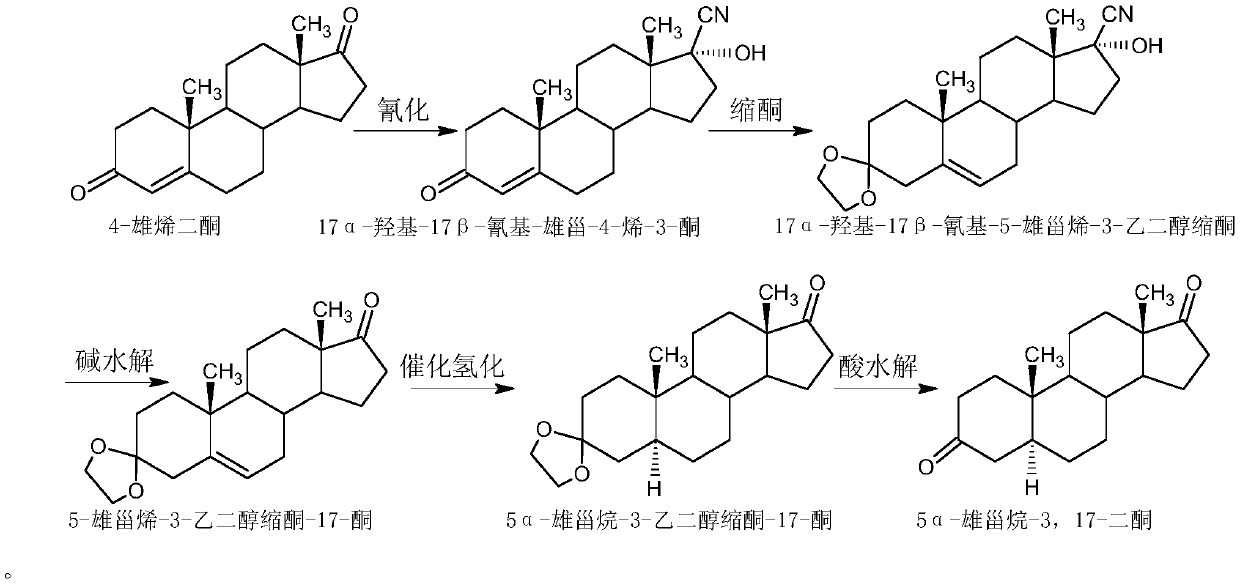
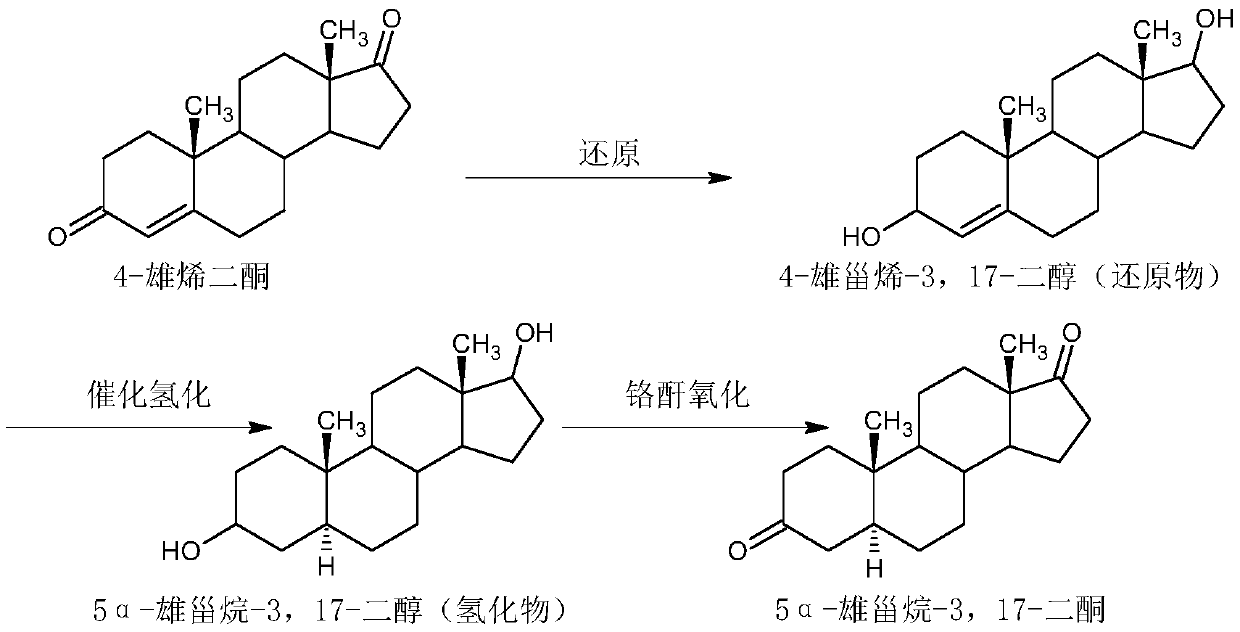
Preparation method of 5 alpha-androstane-3, 17-dione
A technology for androstane and diketone, applied in the field of organic chemical synthesis, can solve the problems of inability to effectively avoid isomer hydrogenation by-products, lack of hormone activity, failure to improve the purity of 5α hydrogenation products, etc., and to reduce the generation of , Guarantee the yield level, improve the effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. The preparation method of 17α-hydroxyl-17β-cyano-androst-4-en-3-one is as follows:
[0041] Add 100g of 4-androstenedione, 100ml of acetone cyanohydrin, 200ml of ethyl acetate and 20ml of triethylamine into the reaction flask, stir well and keep it warm at 60°C to 65°C for 20 hours to react; after the reaction is completed, evaporate to dryness under reduced pressure After all the solvents were added, methanol was added to entrain, and concentrated under reduced pressure to a paste; the temperature was lowered to below 5°C, filtered, and dried to obtain 106g of 17α-hydroxyl-17β-cyano-androst-4-en-3-one, weight Yield 106%.
[0042] 2. The preparation method of 17α-hydroxyl-17β-cyano-5-androstene-3-ethylene glycol ketal is as follows:
[0043] Add 20g of 17α-hydroxy-17β-cyano-androst-4-en-3-one, 0.6g of p-toluenesulfonic acid, 10ml of triethyl orthoformate, 20ml of ethylene glycol and 30ml of dichloromethane into the reaction flask, and stir well And keep warm at 33°...
Embodiment 2
[0050] 1. The preparation method of 17α-hydroxyl-17β-cyano-androst-4-en-3-one is as follows:
[0051] Add 100g of 4-androstenedione, 90ml of acetone cyanohydrin, 220ml of ethyl acetate and 15ml of diethylamine into the reaction bottle, stir well and keep it warm at 60°C to 65°C for 24 hours to react; after the reaction is completed, evaporate to dryness under reduced pressure After all the solvents were added, methanol was added to entrain, and concentrated under reduced pressure to a paste; the temperature was lowered to below 5°C, filtered and dried to obtain 105.9g of 17α-hydroxyl-17β-cyano-androst-4-en-3-one, The weight yield was 105.9%.
[0052] 2. The preparation method of 17α-hydroxyl-17β-cyano-5-androstene-3-ethylene glycol ketal is as follows
[0053] Add 20g17α-hydroxyl-17β-cyano-androst-4-en-3-one, 0.4g sulfosalicylic acid, 8ml triethyl orthoformate, 40ml ethylene glycol and 20ml dichloromethane into the reaction flask, stir After the reaction is completed, cool d...
Embodiment 3
[0060] 1. The preparation method of 17α-hydroxyl-17β-cyano-androst-4-en-3-one is as follows:
[0061] Add 100g of 4-androstenedione, 120ml of acetone cyanohydrin, 180ml of ethyl acetate and 30ml of diethylamine into the reaction bottle, stir well and keep it warm at 55°C to 60°C for 22 hours to react; after the reaction is completed, evaporate to dryness under reduced pressure After all the solvents were added, methanol was added to entrain, and concentrated under reduced pressure to a paste; the temperature was lowered to below 5°C, filtered and dried to obtain 106.5g of 17α-hydroxyl-17β-cyano-androst-4-en-3-one, The weight yield was 106.5%.
[0062] 2. The preparation method of 17α-hydroxyl-17β-cyano-5-androstene-3-ethylene glycol ketal is as follows:
[0063] Add 20g of 17α-hydroxy-17β-cyano-androst-4-en-3-one, 0.8g of p-toluenesulfonic acid, 20ml of triethyl orthoformate, 18ml of ethylene glycol and 40ml of chloroform into the reaction flask, and stir well And keep warm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com