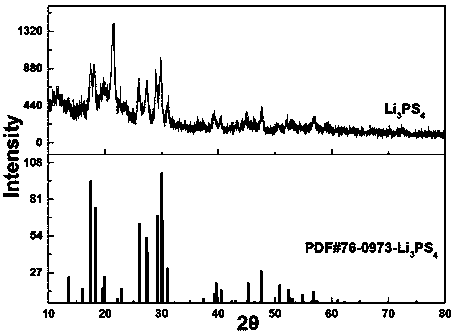
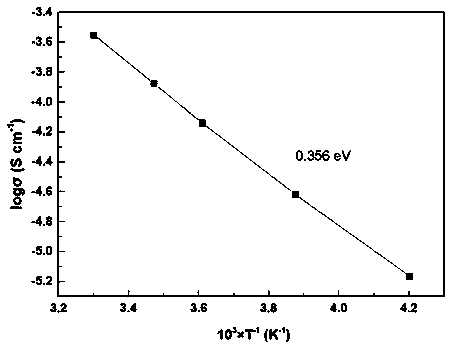
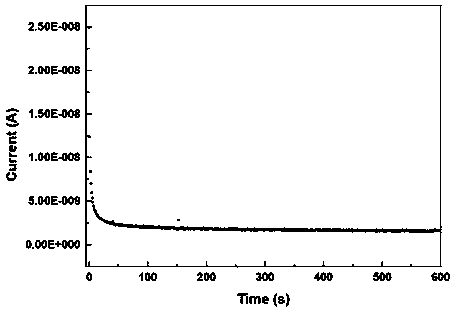
Preparation method of sulfide solid electrolyte
A solid electrolyte and sulfide technology, applied in solid electrolytes, non-aqueous electrolytes, circuits, etc., can solve the problems of unfavorable industrial production and high production costs, and achieve the effects of reducing preparation costs, easy composition, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Step 1: Prepare the anhydrous ethylene glycol dimethyl ether solution of metal lithium-biphenyl: weigh 30.842 g of biphenyl and dissolve it in the corresponding volume of anhydrous ethylene glycol dimethyl ether solvent according to the concentration of 1 mol / L. Benzene is completely dissolved, add 1.3882 g of metal lithium wire with a length of 1 cm and a width of 1 mm, and let it stand at room temperature until it is completely dissolved;
[0022] Step 2: Weigh 3.206 g of elemental sulfur and 7.408 g of phosphorus pentasulfide into the above-mentioned anhydrous ethylene glycol dimethyl ether solution of metal lithium-biphenyl, and stir at 80 °C for 6 h to obtain a suspension;
[0023] Step 3: Separate the suspension from the precipitate and the supernatant with a centrifuge at a speed of 8000 r / min and a centrifugation time of 20 min. The supernatant is recovered for reuse, and the precipitate is centrifugally washed with anhydrous ethylene glycol xylene 3 times, the ...
Embodiment 2
[0026] Step 1: Prepare metal lithium-naphthalene anhydrous tetrahydrofuran solution: weigh 25.636 g of naphthalene and dissolve it in the corresponding volume of anhydrous tetrahydrofuran solvent at a concentration of 2 mol / L. After the biphenyl is completely dissolved, add 1.3882 g of 2 mm, 0.5 mm thick lithium metal, let it stand until it is completely dissolved;
[0027]Step 2: Weigh 3.206 g of elemental sulfur and 9.525 g of phosphorus pentasulfide and add the above-mentioned lithium-naphthalene anhydrous tetrahydrofuran solution, and stir at room temperature for 8 h to obtain a suspension;
[0028] Step 3: Use a centrifuge to separate the suspension from the precipitate and the supernatant at a speed of 8000 r / min, and the centrifugation time is 20 min. The supernatant is recovered for reuse. The precipitate is centrifuged and washed 3 times with anhydrous THF at The centrifugation time was 6000 r / min, and the centrifugation time was 10 min; then the precipitate was dried...
Embodiment 3
[0030] Step 1: Prepare the anhydrous ethylene glycol dimethyl ether solution of metal lithium-biphenyl: weigh 30.842 g of biphenyl and dissolve it in the corresponding volume of anhydrous ethylene glycol dimethyl ether solvent according to the concentration of 1 mol / L. Benzene is completely dissolved, add 1.3882 g of metal lithium wire with a length of 1 cm and a width of 1 mm, and let it stand at room temperature until it is completely dissolved;
[0031] Step 2: Weigh 1.603 g of elemental sulfur and 11.112 g of phosphorus pentasulfide into the above-mentioned anhydrous ethylene glycol dimethyl ether solution of metal lithium-biphenyl, and stir at 80 °C for 6 h to obtain a suspension;
[0032] Step 3: Separate the suspension from the precipitate and the supernatant with a centrifuge at a speed of 8000 r / min and a centrifugation time of 20 min. The supernatant is recovered for reuse, and the precipitate is centrifugally washed with anhydrous ethylene glycol xylene 3 times, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com