Application of protein in preparation of medicine for preventing and treating atherosclerosis and complications
A technology for atherosclerosis and atherosclerosis, which is applied in the field of biomedicine, can solve the problems of limited research level and no drugs for oxLDL clearance, and achieve the effect of reducing the ratio level.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
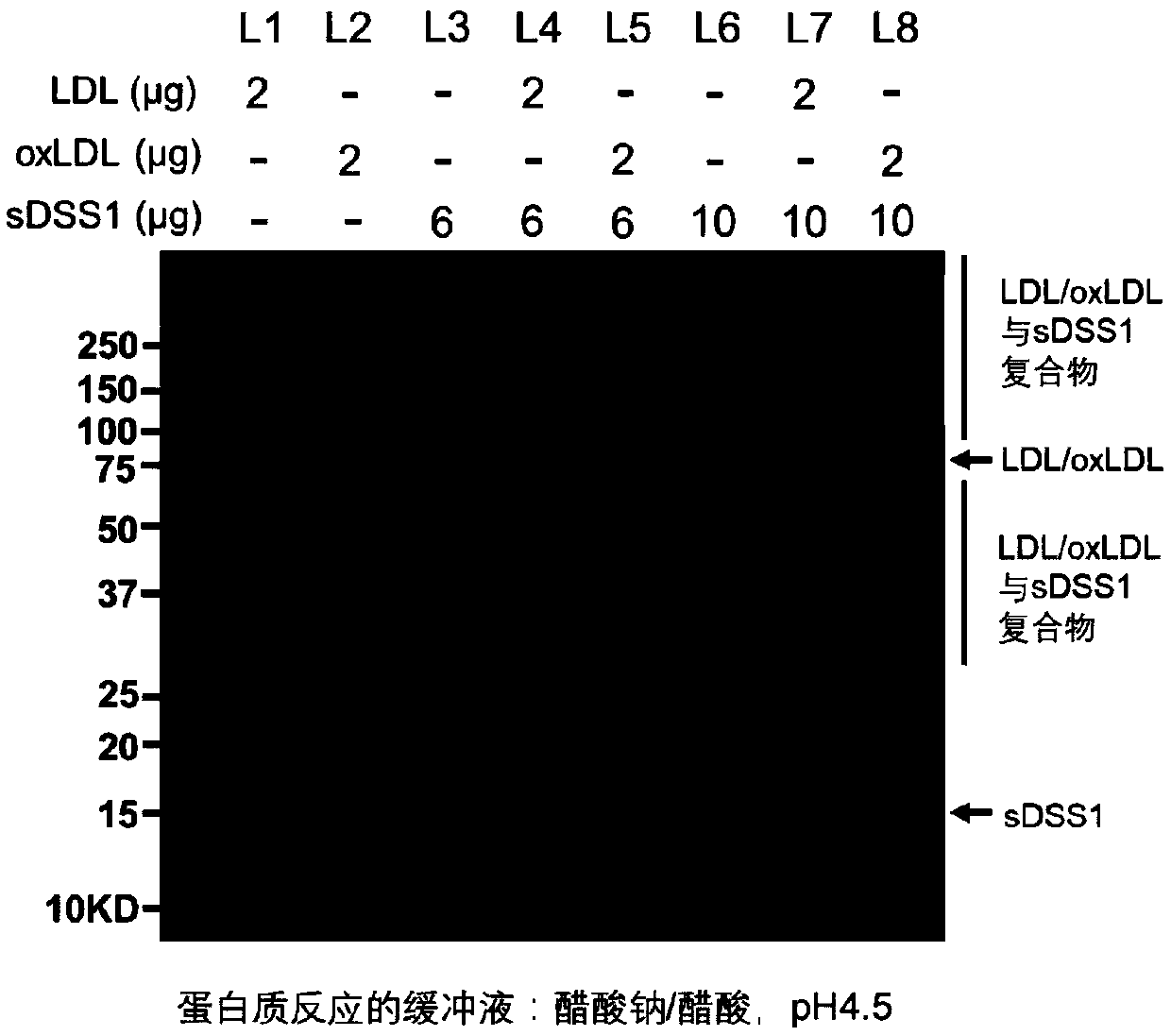
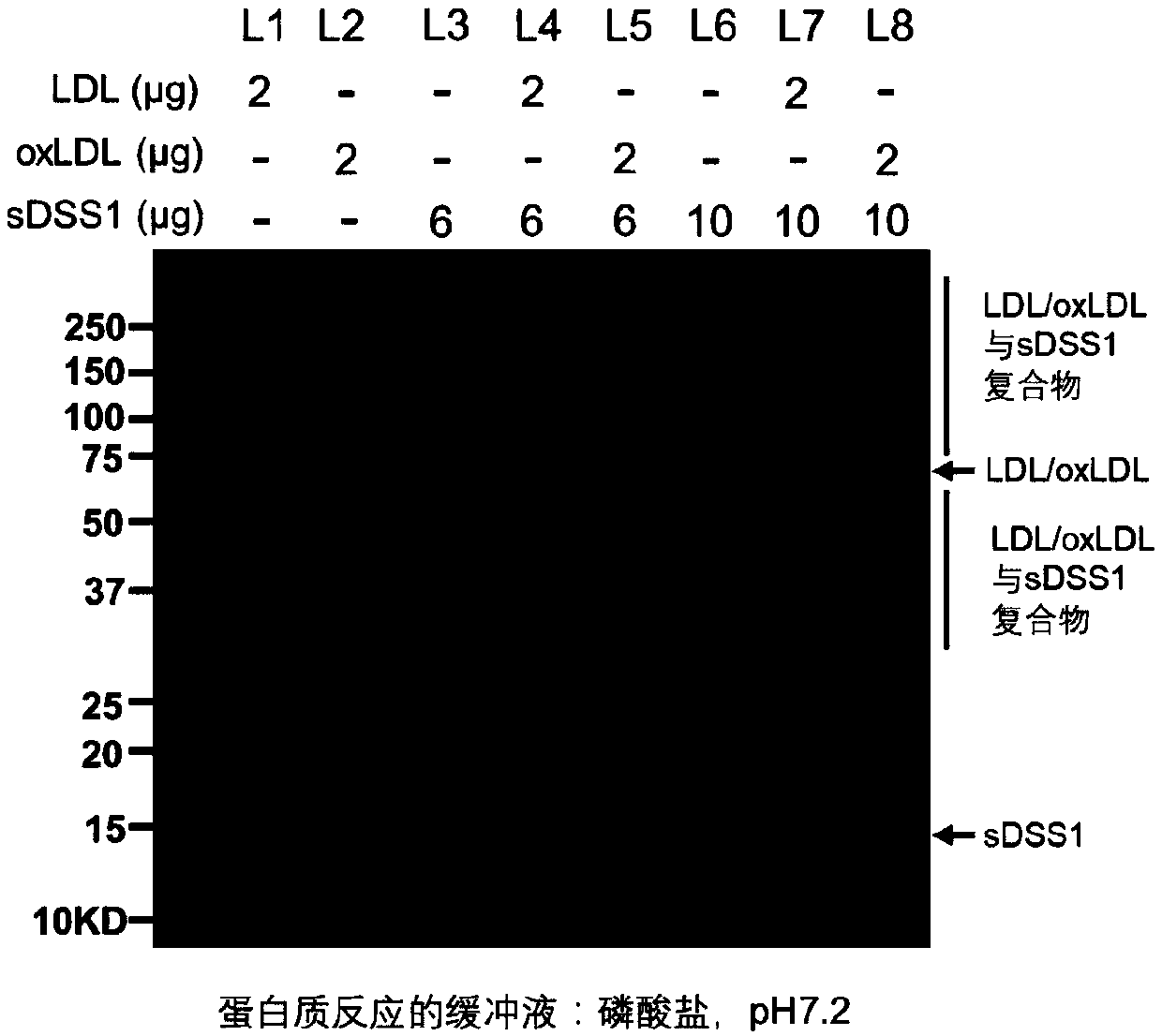
[0114] Example 1. sDSS1 protein can interact with oxLDL or LDL.
[0115] 1.1 Experimental materials and methods
[0116] Experimental materials: sDSS1 protein, oxidized low-density lipoprotein (oxLDL) (Sulaibao, product number: H7980); low-density lipoprotein (LDL) (Solaibao, product number: H7960).
[0117] Experimental method: Add 2μg of oxLDL or LDL and 6μg of sDSS1 protein or 10μg of sDSS1 protein to 1.5ml under the conditions of 20mM sodium acetate / acetic acid buffer (pH4.5) or 20mM phosphate buffer (pH7.2) respectively Mix in the EP tube and incubate at 37°C for 12 hours. The incubation product was added with loading buffer, mixed evenly, and denatured at 100°C for 10 minutes to make a loading sample. The prepared samples were electrophoretically separated by polyacrylamide gel electrophoresis (SDS-PAGE). After separation, the SDS-PAGE gel was stained with Coomassie brilliant blue to display protein bands.
[0118] 1.2 Experimental results
[0119] In the acetate buf...
Embodiment 2
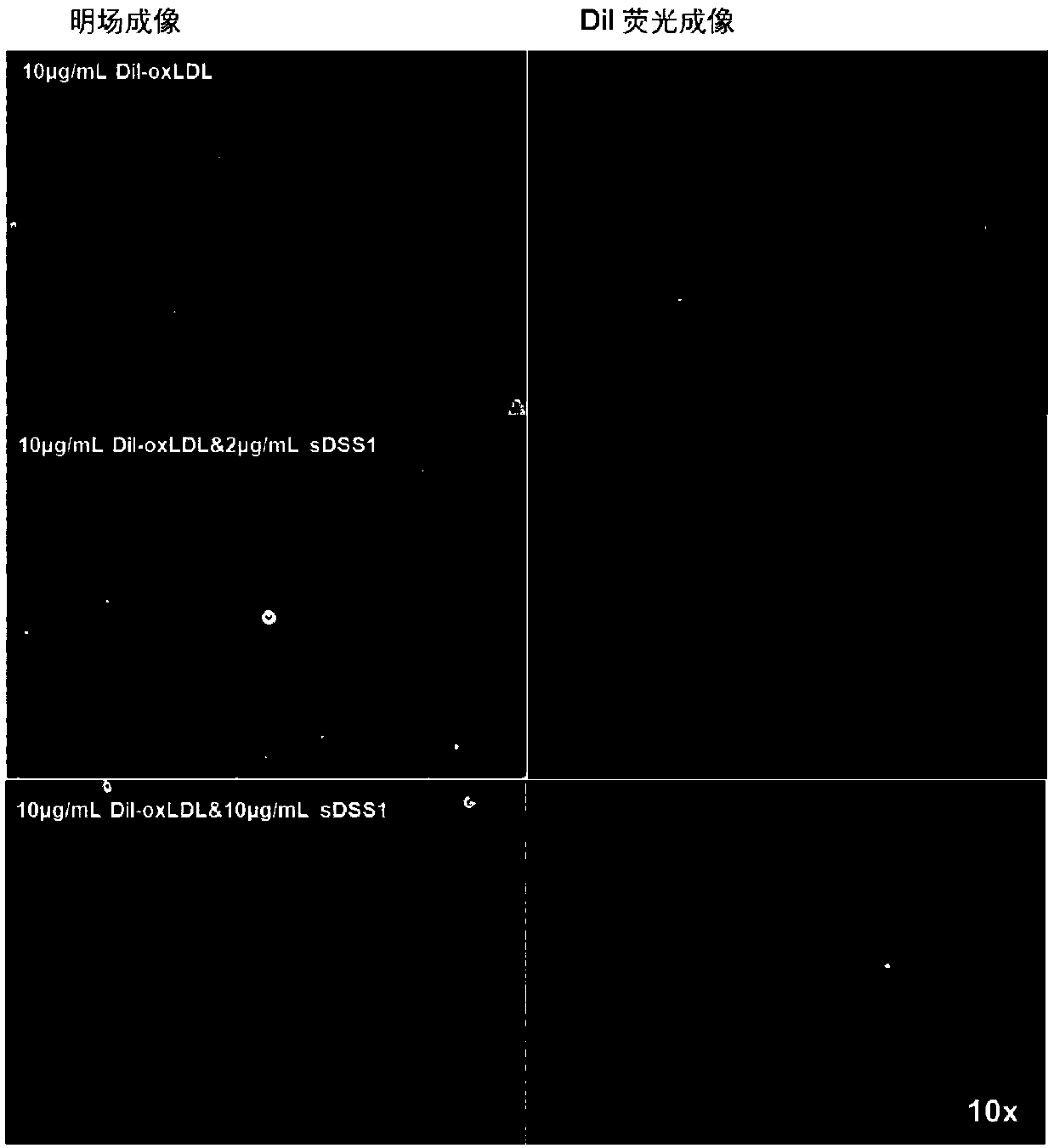
[0122] Example 2. sDSS1 protein can reduce the uptake of oxLDL by vascular endothelial cells.
[0123] 2.1 Experimental materials and methods
[0124]Experimental materials: sDSS1 protein, Dil-oxLDL (Thermo Fisher Scientific, catalog number: L34358), human umbilical vein endothelial cells (HUVEC) (PromoCell, catalog number: C-12200)
[0125] Experimental method: HUVEC cells were inoculated into 6-well plates according to the number of cells of 300,000 per well. After 24 hours of attachment, 1.5ml of 10μg / ml Dil-oxLDL was added, or 1.5ml of 10μg / ml Dil-oxLDL was added at the same time at a concentration of 2 μg / ml, 5 μg / ml, 10 μg / ml, 20 μg / ml of sDSS1 protein. After continuing to incubate in the incubator for 5 hours, the fluorescent signal in the cells was observed using a fluorescence microscope, and the cells were digested with trypsin to form single cells for flow cytometry to detect the fluorescence intensity.
[0126] 2.2 Experimental results
[0127] After adding Dil-...
Embodiment 3
[0128] Example 3. sDSS1 protein inhibits phagocytosis of oxLDL by macrophages.
[0129] 3.1 Experimental materials and methods
[0130] Experimental materials: sDSS1 protein, Dil-oxLDL, phorbol ester (PMA, Sigma-Aldrich Company, product number: P1585), human monocyte THP-1 (Cell Bank of Type Culture Collection Committee, Chinese Academy of Sciences, catalog number: SCSP- 567).
[0131] Experimental method: THP-1 cells were inoculated into 6-well plates according to the number of 250,000 cells per well, and the culture medium contained 100ng / mL PMA. After the cells were activated by PMA for 48 hours, they were replaced with fresh medium and cultured for four days to help the cells adhere to the wall. Discard the old medium, add 1.5ml 10μg / ml Dil-oxLDL, or add 1.5ml 10μg / ml Dil-oxLDL and add sDSS1 protein at the concentration of 2μg / ml, 5μg / ml, 10μg / ml, 20μg / ml. After continuing to incubate in the incubator for 5 hours, observe the fluorescent signal in the cells with a fluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com