Application of Obatoclax in preparation of HIV-1 latent infection reversal agent
A technology of HIV-1 and latent infection, applied in the field of medicine, can solve the problems of restricting wide application, poor specificity of action, large toxic and side effects, etc., and achieve the effect of promoting death and preventing supplementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
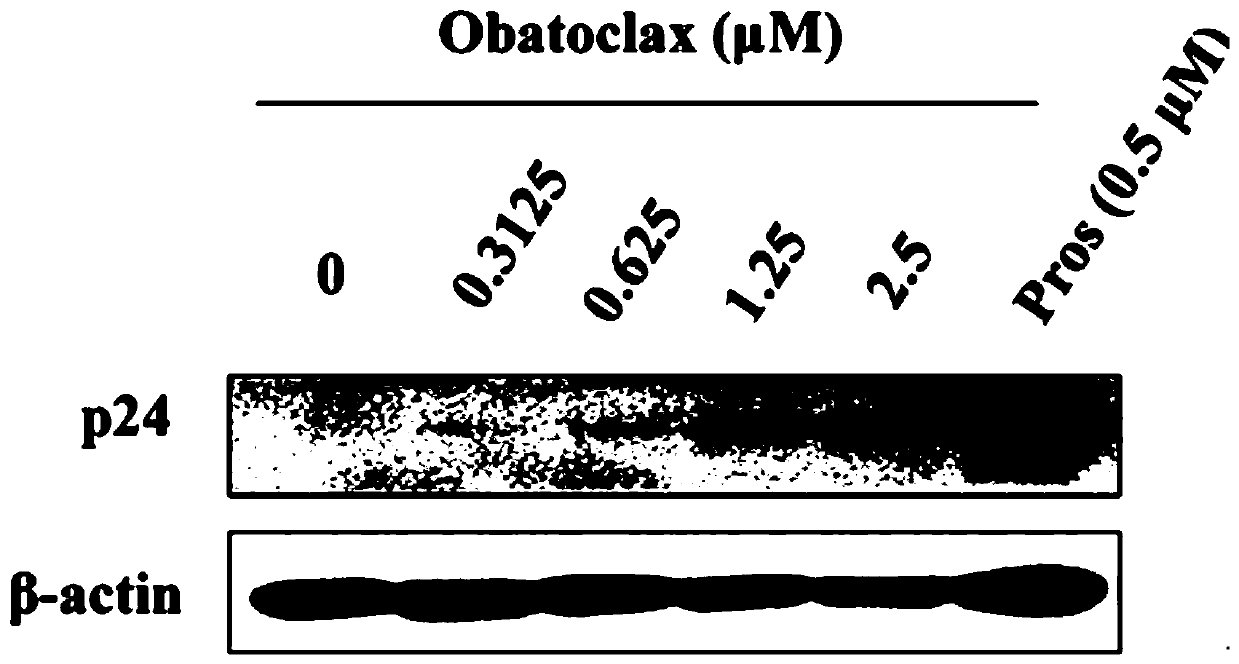
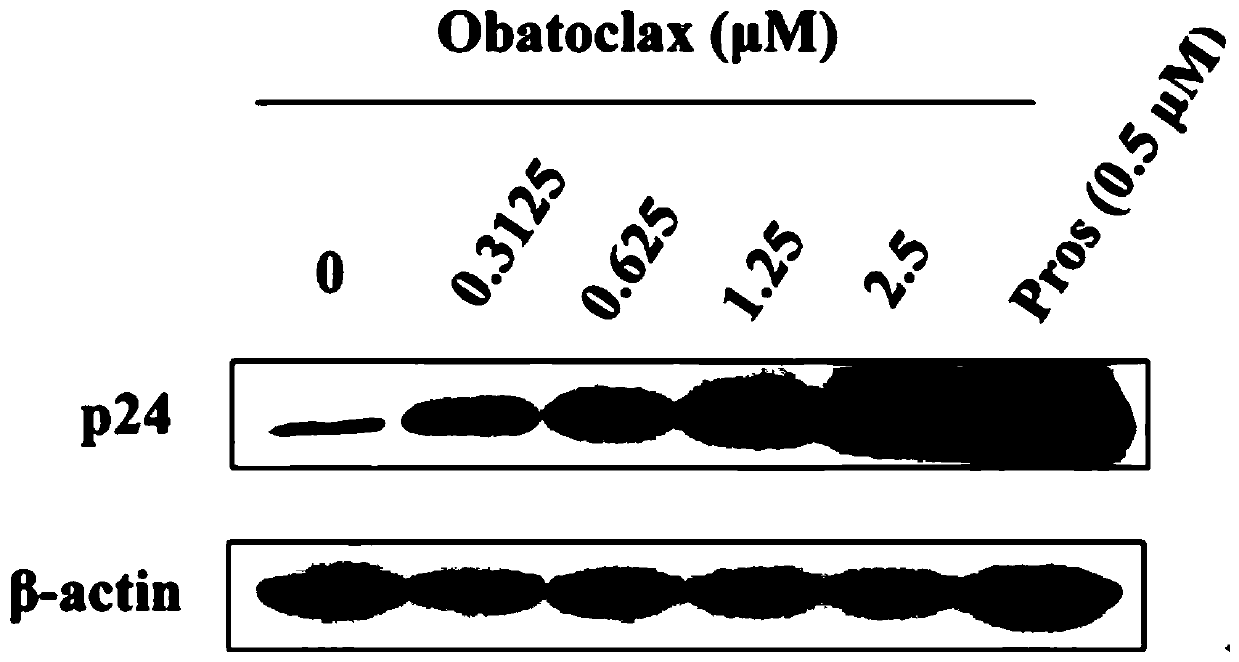
[0031] In Example 1, the effect of Obatoclax on the expression of P24 protein in HIV-1 latent infection cell model J-Lat 10.6 and Ach2 cells was studied, and it was proved that Obatoclax can effectively promote the expression of P24 protein in J-Lat 10.6 and Ach2 cells. The specific process is as follows:
[0032] (1) Culture and activation of HIV-1 latently infected cell lines
[0033] 1) Collect J-Lat 10.6 and Ach2 cell liquid in a centrifuge tube, centrifuge at 800rpm for 5min, discard the supernatant, and keep the cell pellet.
[0034] 2) Add 5ml of culture medium, blow the cells evenly, pipette 20μl of cell fluid into the cell counting plate, and count.
[0035] 3) Adjust the cell concentration to 1×10 6 cells / ml, spread in 6-well plate at 2ml / well.
[0036] 4) Add serial dilutions of different concentrations of Obatoclax (0-2.5 μM) and the corresponding positive drug Prostratin.
[0037] 5) At 37°C, with 5% CO 2 After incubation in the incubator for 48 h, the cells ...
Embodiment 2
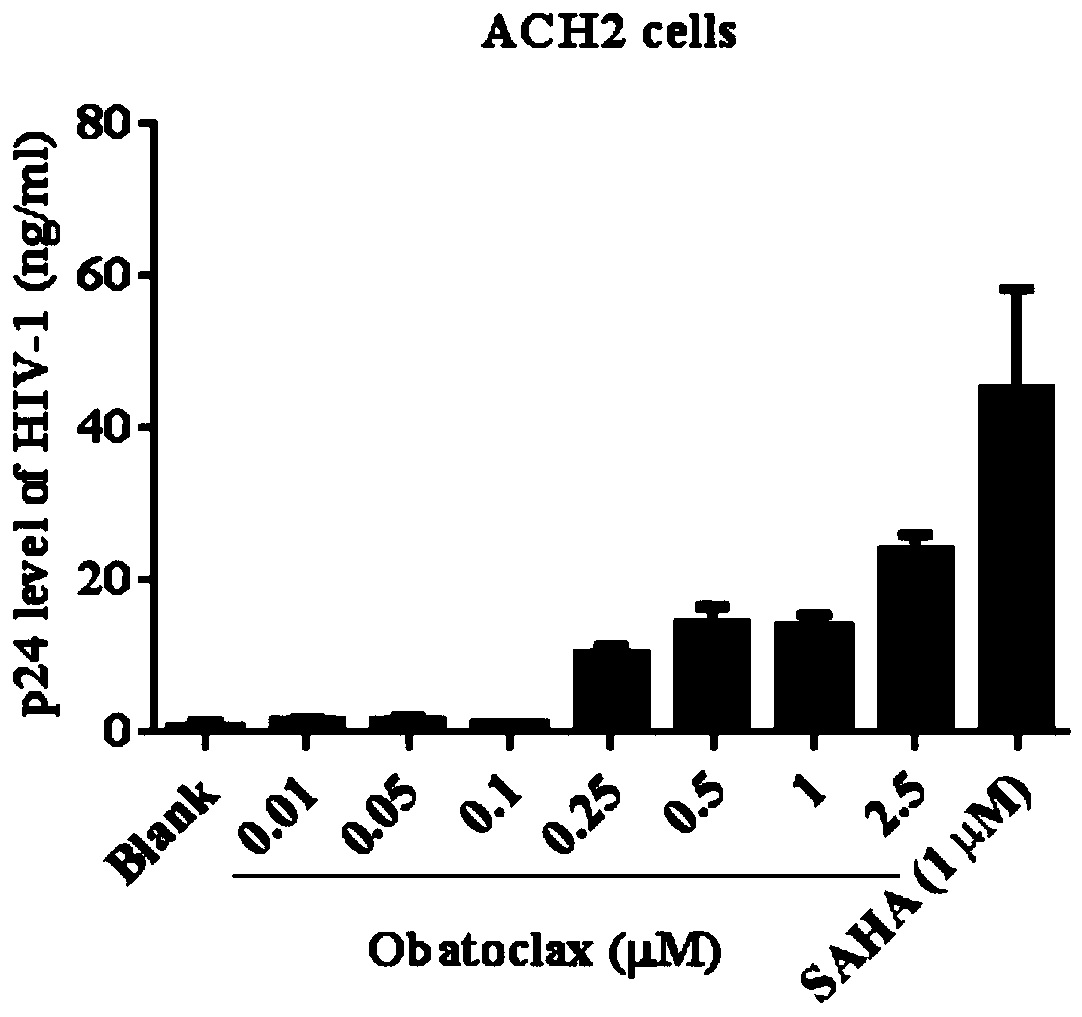
[0060] The present embodiment detects the effect of Obatoclax on the HIV-1p24 protein expression of HIV-1 latently infected cell model ACH2 cells by enzyme-linked immunosorbent assay (ELISA), and the specific test process is as follows:
[0061] 1) Collect the ACH2 cell liquid in a centrifuge tube, centrifuge at 800rpm for 5min, discard the supernatant, and keep the cell pellet.
[0062] 2) Add 5ml of culture medium, blow the cells evenly, pipette 20μl of cell fluid into the cell counting plate, and count.
[0063] 3) Adjust the cell concentration to 1×10 6 cells / ml, spread in 96-well plate at 100 μl / well.
[0064] 4) Add serially diluted different concentrations of Obatoclax (0.01-2.5 μM).
[0065] 5) At 37°C, 5% CO 2 After incubation in the incubator for 48 hours, 100 μl of cell culture supernatant was collected and placed in another 96-well plate, an equal volume of 5% Triton X-100 was added, and incubated overnight at 4°C.
[0066] 6) Use ELISA p24 protein detection re...
Embodiment 3
[0069] In this example, the cytotoxicity of Obatoclax to J-Lat 10.6, ACH2, and J-Lat A2 latently infected cell lines was analyzed by the MTT method. The specific operations are as follows:
[0070] 1) Collect J-Lat 10.6, ACH2, J-Lat A2 cell fluid in a centrifuge tube at 1×10 6 Each / ml, 100μl / well was added to a 96-well cell culture plate;
[0071] 2) Add Obatoclax prepared in serially diluted blank culture medium, 100 μl / well.
[0072] 3) At 37°C, 5% CO 2 After incubating in the incubator for 48h, add MTT solution (5mg / ml, prepared in PBS, pH=7.4) 20μl / well, continue at 37°C, 5%CO 2 Incubate for 4h. Centrifuge at 3000rpm for 5min, carefully discard the culture supernatant in the well, add 100μl DMSO to each well, and shake for 15min to dissolve the crystals;
[0073] 4) Use a microplate reader to detect the absorbance at 570 nm in each well.
[0074] The result is as Figure 4~6 , which in turn represent the cytotoxicity of Obatoclax to J-Lat 10.6, ACH2, J-Lat A2 cells, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com