HPLC fingerprint detection method of kidney-tonifying and bone-strengthening traditional Chinese medicine
A technology of fingerprint, invigorating kidney and strengthening bones, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of time-consuming and labor-intensive detection, threat to drug safety, low efficiency, etc., to achieve convenient operation, avoid adulteration of traditional Chinese medicine, use Instrument-less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Instruments and reagents
[0039] DIONEX P680 high performance liquid chromatograph, quaternary pump, online vacuum degassing system, autosampler, column thermostat, UV detector; AB204-E electronic balance (Shanghai Mettler-Toledo Instrument Co., Ltd.).
[0040] 10 batches of Bushen Jiangu Capsules (190201-03, 190404-05, 190706-10, a total of 10 batches); icariin reference substance (for content testing, purchased by China Institute for Food and Drug Control, batch number: 110737- 200415); acetonitrile was chromatographic grade, water was purified water, and the rest were chemical grade.
[0041] 2. Method steps
[0042] (1) Preparation of the test solution: Grind the contents of the Bushen Jiangu Capsules to be tested finely, take 1.5g, accurately weigh, add 30ml of 50% methanol accurately, weigh, ultrasonic 30 minutes (power 250W, frequency 40kHz), let it cool, and then weigh it, make up the lost weight with 50% methanol, shake well, filter, and take the filtrate...
Embodiment 2
[0048] Embodiment 2 detection condition optimization
[0049] 1. Preparation of the test solution
[0050] According to the prescription and preparation characteristics of Bushen Jiangu Capsules, 50% methanol and 50% ethanol were respectively investigated as extraction solvents to prepare the test solution. Refer to Example 1 for specific methods.
[0051] Column: Agilent ZORBAX SB-C 18 , 4.6×250mm, 5um; detection wavelength: 235nm; injection volume 10ul; column temperature: 30°C; flow rate: 1ml / min; mobile phase A: acetonitrile, mobile phase B: containing 0.1% potassium dihydrogen phosphate and 0.05% octane Aqueous solution of sodium alkanesulfonate, the gradient elution program is shown in Table 1.
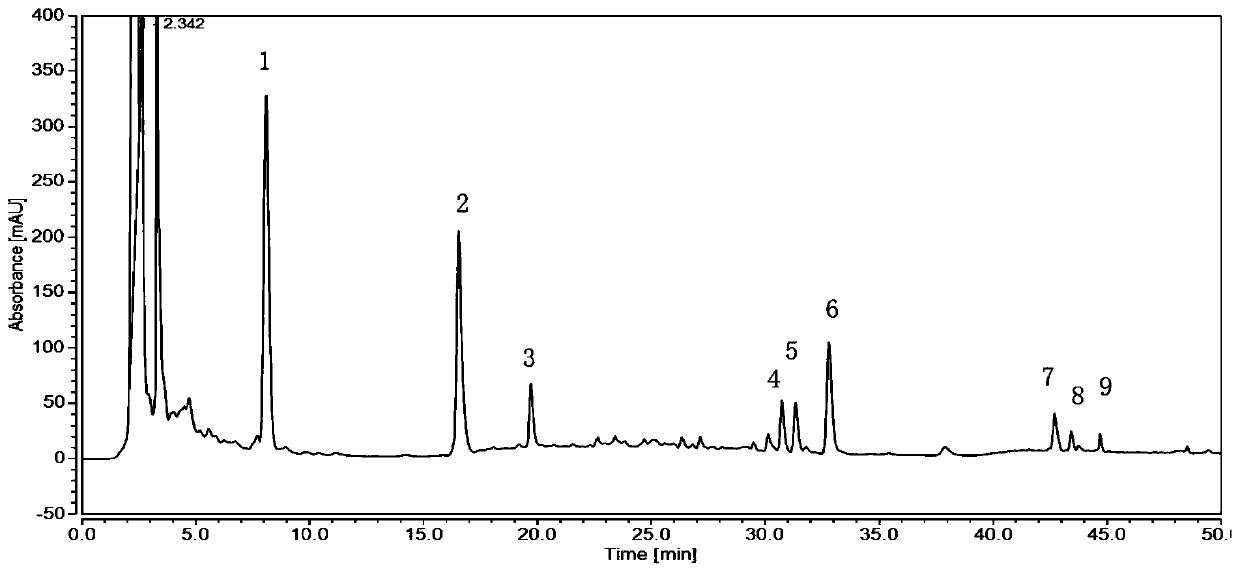
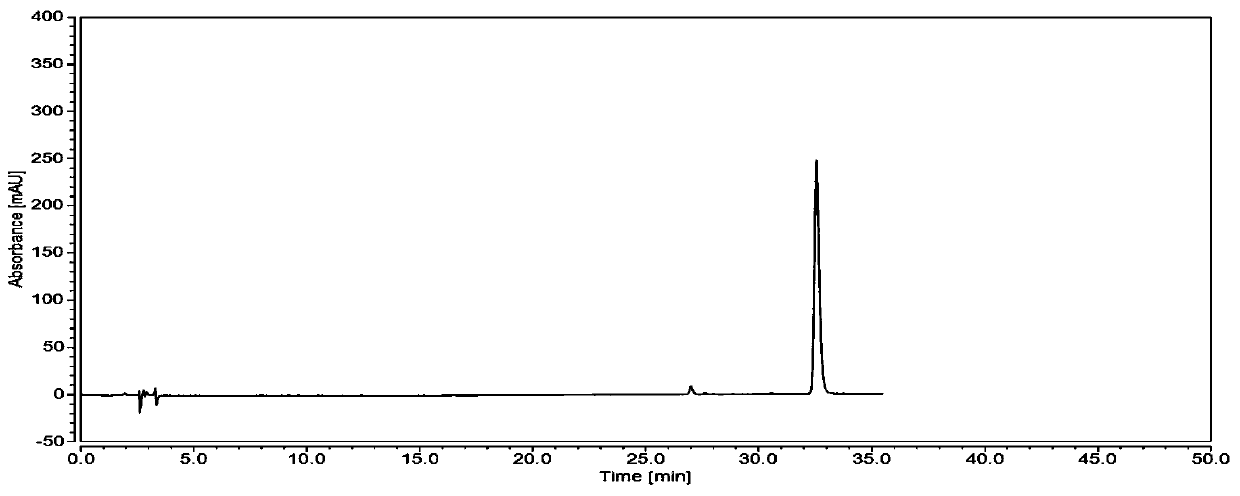
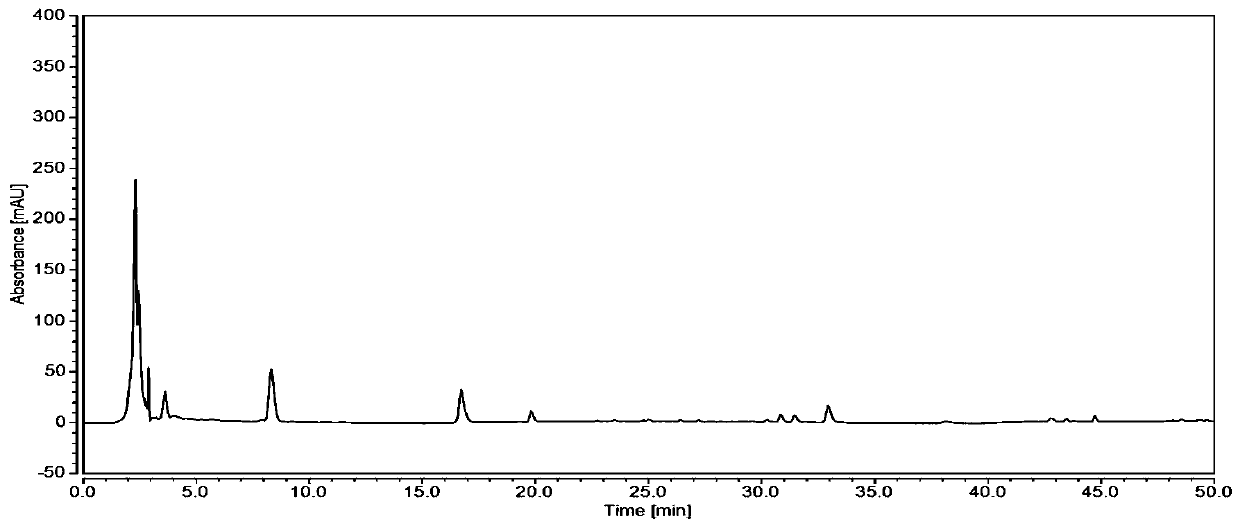
[0052] From figure 1 and image 3 It can be seen that the chromatographic peak area of the resulting test solution extracted by 50% methanol is larger, and the separation effect of the three peaks in 40-45 minutes is better, so it is determined that 50% methanol is used as ...
Embodiment 3
[0060] Embodiment 3 methodological investigation
[0061] 1. Stability test
[0062] Get the test solution according to the above-mentioned chromatographic conditions, measure at 0, 4, 8, 12h, 24h, investigate the relative retention time of each common peak and the RSD of the relative peak area. The results show that the relative retention time of each chromatographic peak is less than 2.0%, and the relative peak area of each chromatographic peak is less than 2.0%, which meet the technical requirements of fingerprint. See Table 3-4.
[0063] Table 3 Bushen Jiangu Capsule Stability Investigation Relative Retention Time
[0064]
[0065] Table 4 Relative peak area of Bushen Jiangu Capsule Stability Investigation
[0066]
[0067] 2. Repeatability test
[0068] Get 6 parts of samples of the same batch number, prepare 6 parts of need testing solution according to the preparation method of need testing solution under item 1, measure under the above-mentioned chromatog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com