Ten-medicine Xiang Lu capsule, preparation method thereof and ginsenoside content analysis method
A technology of ginsenoside and fragrant deer, which is applied in the field of verification of traditional Chinese medicine and its efficacy, can solve the problems of long cycle and inconspicuous healing effect, and achieve the effects of reducing the amount of use, increasing the activity of gonads, and promoting biosynthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
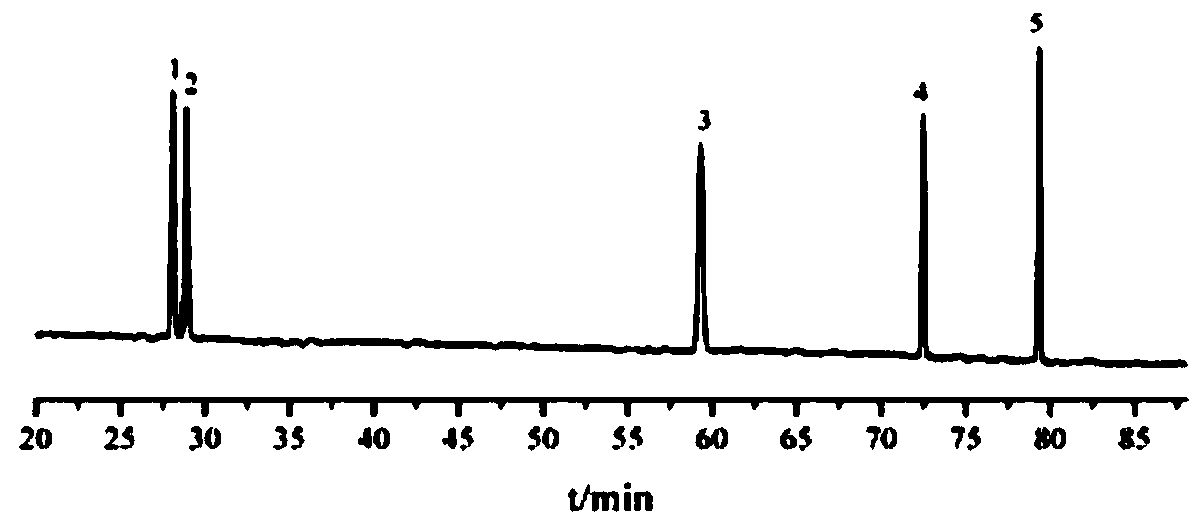
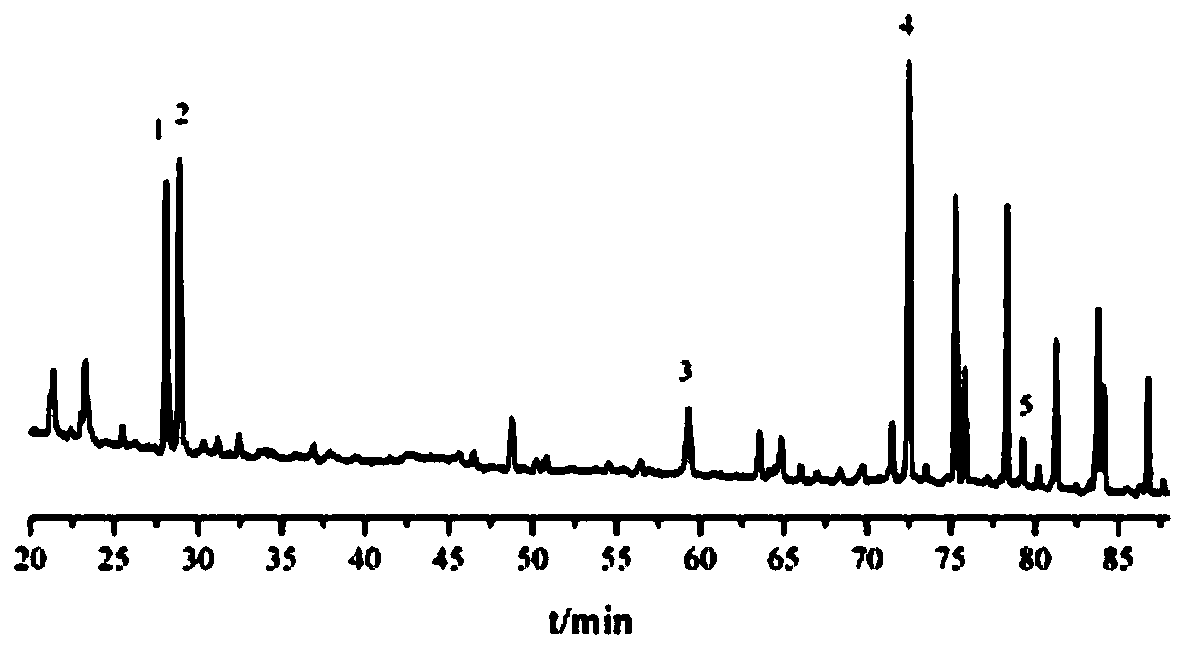
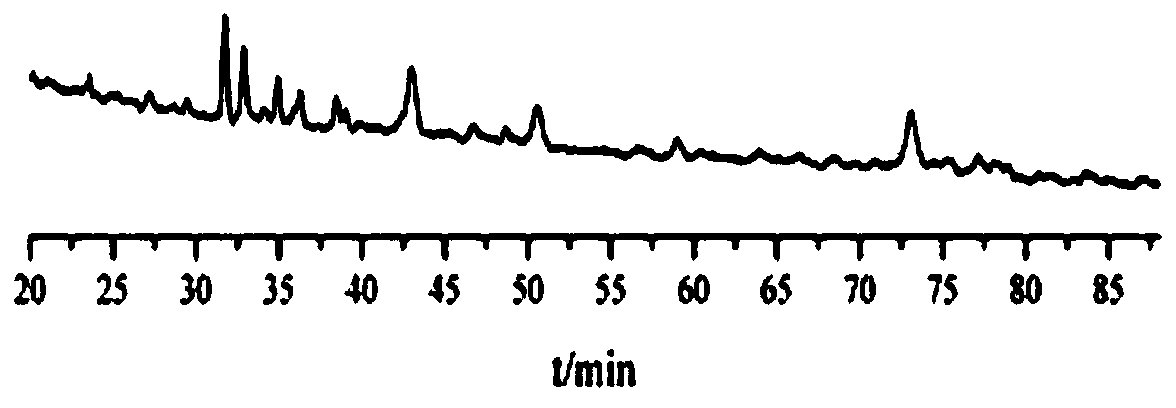
Image
Examples
Embodiment Construction
[0036]Shiwei Xianglu Capsules, according to the ratio of parts by mass, includes 10-30 parts of Cyperus cyperi, 2-6 parts of Allium scallops, 2-6 parts of deer antlers, 2-6 parts of fritillary fritillaria, 2-6 parts of curcuma, centipede, 2 to 6 servings of winter, 2 to 6 servings of turtle shell (burnt), 2 to 6 servings of raw sun-dried ginseng, and 1 to 3 servings of bellflower. The optimal ratio is 10 parts of Cyperus cyperi, 6 parts of scallops, 6 parts of deer antlers, 6 parts of fritillaria, 6 parts of curcuma, 1 part of centipede, 6 parts of asparagus, 6 parts of turtle shell (burned) and 6 parts of ginseng And bellflower 3 servings.
[0037] The preparation method of Shiwei Xianglu Capsules comprises the following steps, and the following steps are carried out in sequence,
[0038] Step 1, according to the proportioning described in claim 1, take the centipede and 0.5 times the raw sun-dried ginseng in the formula and grind it into a fine powder with a particle size o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com