Hydrogenation catalyst for doxycycline production and preparation method and application thereof
A hydrogenation catalyst, doxycycline technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, catalytic reaction, etc. and other problems, to achieve the effect of easy recovery, good gas-liquid distribution, and reduction of isomerization reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
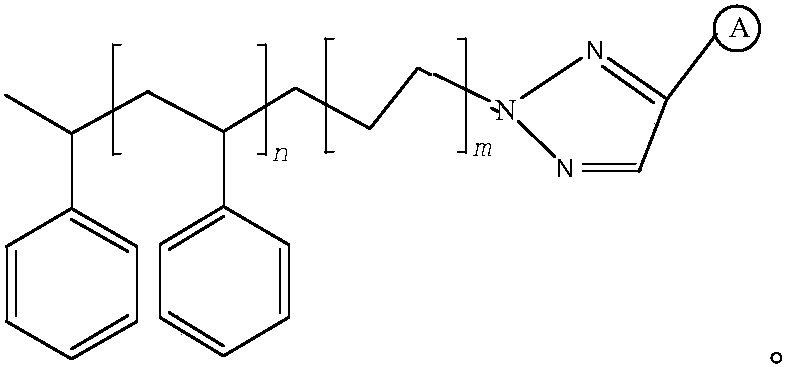
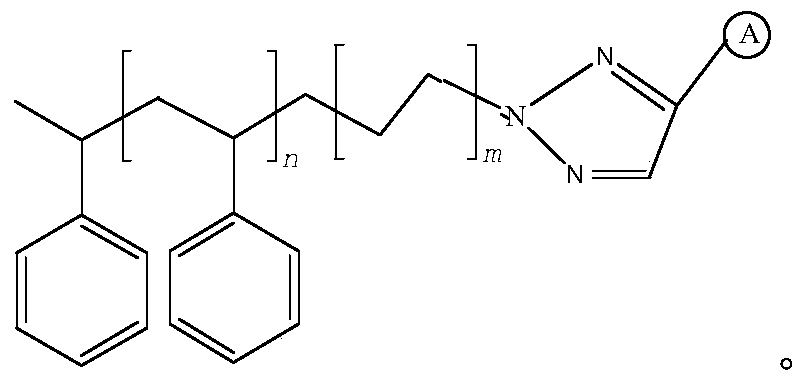
[0027] put R 1 -N 3 (R 1 for polystyrene) with R 2 -CH≡CH(R 2 is the ligand binap) in anhydrous THF (tetrahydrofuran) solvent with cuprous chloride as a catalyst to stir until the reaction is completed, filter dry and wash with alcohol before use.
[0028] Stir 1kg of polystyrene load (n=500, m=1, and the modification group is binap) in 20kg of 42% ethanol, add a hydrochloric acid solution containing 0.02kg of palladium chloride and stir, stir for 1 hour, stir at a temperature of 25°C, and cool After reaching 10°C, add 9kg of 11α-chlorometacycline p-toluenesulfonate, pass hydrogen to react for 7h, filter after completion of the reaction, and separate the palladium-polystyrene supported catalyst and the reaction solution.
[0029] Compared with the results of using Pd / C as catalyst under the same conditions, the relative content of doxycycline was increased by 6.7%, the relative content of 6-epidoxycycline was decreased by 42.7%, and the yield of doxycycline was increased b...
Embodiment 2
[0031] 1kg polystyrene load (n=500, m=3, the modification group is quinoline, the preparation method is the same as that of Example 1, R 2 For ligand quinoline), stir in 20kg 42% ethanol, add hydrochloric acid solution containing 0.02kg palladium chloride and stir, stir for 1 h, stirring temperature is 25 ℃, after cooling to 10 ℃, add 9kg 11α-chlorometacycline The p-toluenesulfonate was passed through hydrogen to react for 8 hours, and after the reaction was completed, the palladium-polystyrene supported catalyst and the reaction solution were separated by filtration.
[0032] Compared with the results of using Pd / C as catalyst under the same conditions, the relative content of doxycycline was increased by 6.1%, the relative content of 6-epidoxycycline was decreased by 35.3%, and the yield of doxycycline was increased by 2.0%.
Embodiment 3
[0034] Put 1kg of the polystyrene load (n=500, m=3, the modification group is quinoline) filter cake obtained in Example 2 into a chromatographic column with an inner diameter of 15cm, rinse with 200mL of ethanol and 200mL of purified water, and then use 1.7 L 0.3mol / L thiourea and 0.2mol / L hydrochloric acid aqueous solution were washed, and the washing solution was collected. The washing solution was adsorbed and recovered by cation exchange resin and then eluted with hydrochloric acid to obtain PdCl 2 The recovery rate of palladium is 99.1%; the recovered thiourea can be recycled and used in the next round of catalyst recovery process. The eluted polystyrene load was soaked in ammonia water, and rinsed with water and ethanol respectively for use after rinsing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com