Method for preparing fulvestrant and intermediates
A fulvestrant and intermediate technology, which is applied in the field of pharmaceutical synthesis, can solve the problems of low yield, high route cost and high energy consumption, and achieves the effects of high yield, safe reaction reagents and high low-temperature conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
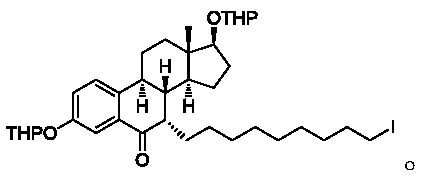
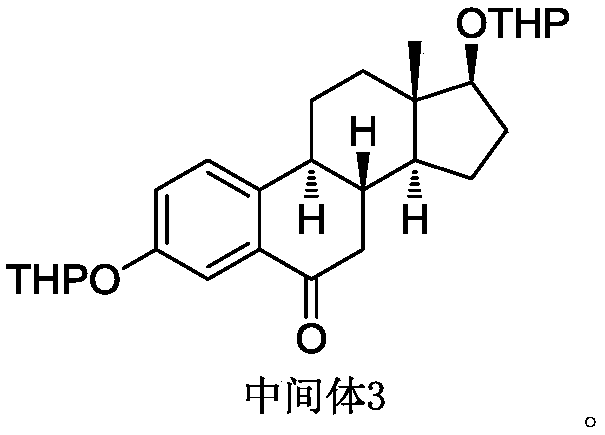
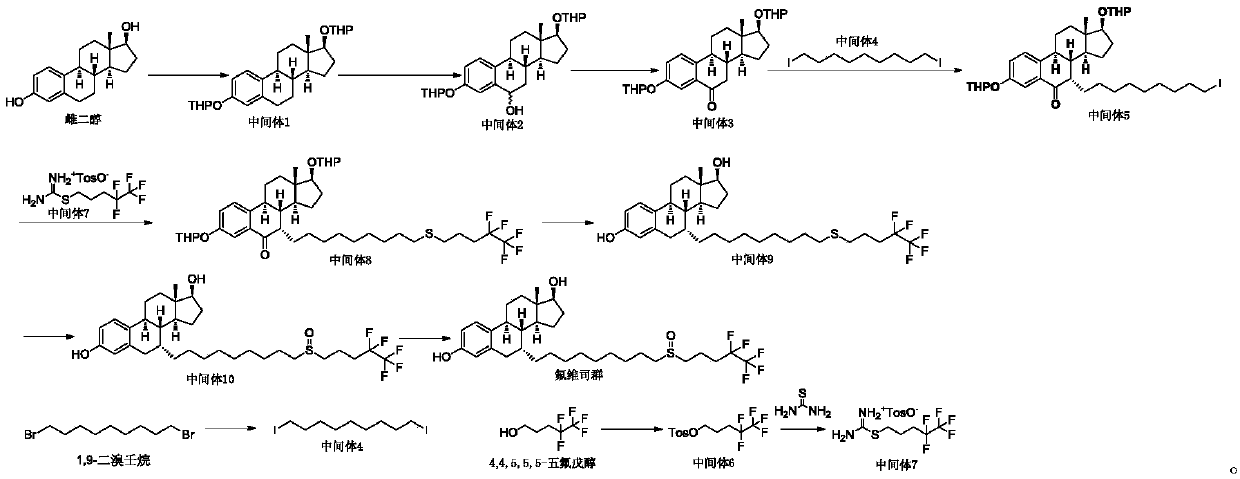
[0037] Intermediate 3 (30 g, 66 mmol, 1 eq) and toluene (150 ml, 5V) were added to the reaction flask. Add potassium tert-butoxide (7.4 g, 66 mmol, 1 eq) and stir for 15 minutes. Add 1,9-diiodononane (50.2g, 132mmol, 2eq) and potassium tert-butoxide (11.1g, 99mmol, 1.5eq) to the reaction solution, and stir at a temperature of 25°C until TLC detects that the reaction is complete (about 8- 10 hours). TLC conditions: PE / EA=10:1, color development with phosphomolybdic acid. Add the reaction solution into saturated ammonium chloride solution (1000ml), stir for 30 minutes, let stand to separate layers, extract the aqueous phase with 1000ml of ethyl acetate, combine the organic phases, wash with 1000ml of water, 1000ml of brine successively, anhydrous sodium sulfate 100 g dry. Filter and concentrate under reduced pressure to obtain 90 g of dark red oil.
[0038] Add 1000ml of ethyl acetate to dissolve, 540g of silica gel to make sand, and 3000g of silica gel for colum...
Embodiment 2
[0040]
[0041]Intermediate 3 (30 g, 66 mmol, 1 eq) and toluene (150 ml, 5V) were added to the reaction flask. Add potassium tert-butoxide (7.4 g, 66 mmol, 1 eq) and stir for 15 minutes. Add 1,9-diiodononane (75.3g, 198mmol, 3eq) and potassium tert-butoxide (11.1g, 99mmol, 1.5eq) to the reaction solution, and stir at a temperature of 25°C until TLC detects that the reaction is complete (about 8 to 10 hours). TLC conditions: PE / EA=10:1, color development with phosphomolybdic acid. Add the reaction solution into saturated ammonium chloride solution (1000ml), stir for 30 minutes, let stand to separate layers, extract the aqueous phase with 1000ml of ethyl acetate, combine the organic phases, wash with 1000ml of water, 1000ml of brine successively, anhydrous sodium sulfate 100 g dry. Filter and concentrate under reduced pressure to obtain 120 g of dark red oil.
[0042] Add 1500ml ethyl acetate to dissolve, 600g silica gel to make sand, and 3000g silica gel to pack. It was...
Embodiment 3
[0044]
[0045] Intermediate 3 (30 g, 66 mmol, 1 eq) and toluene (150 ml, 5V) were added to the reaction flask. Add sodium tert-butoxide (6.4 g, 66 mmol, 1 eq) and stir for 15 minutes. Add 1,9-diiodononane (50.2g, 132mmol, 2eq) and sodium tert-butoxide (9.5g, 99mmol, 1.5eq) to the reaction solution, and stir at a temperature of 25°C until TLC detects that the reaction is complete (about 8~ 10 hours). TLC conditions: PE / EA=10:1, color development with phosphomolybdic acid. Add the reaction solution into saturated ammonium chloride solution (1000ml), stir for 30 minutes, let stand to separate layers, extract the aqueous phase with 1000ml of ethyl acetate, combine the organic phases, wash with 1000ml of water, 1000ml of brine successively, anhydrous sodium sulfate 100 g dry. Filter and concentrate under reduced pressure to obtain 85 g of dark red oil.
[0046] Add 1000ml of ethyl acetate to dissolve, 400g of silica gel to make sand, and 3000g of silica gel for column packi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com