NAMPT lentiviral vector and biological agent and application thereof
A technology of lentiviral vector and lentivirus, which is applied in the direction of reverse transcription RNA virus, virus/bacteriophage, and the introduction of foreign genetic material by using vectors. Effect of NAD+ levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The construction of embodiment 1phNAMPT lentiviral vector
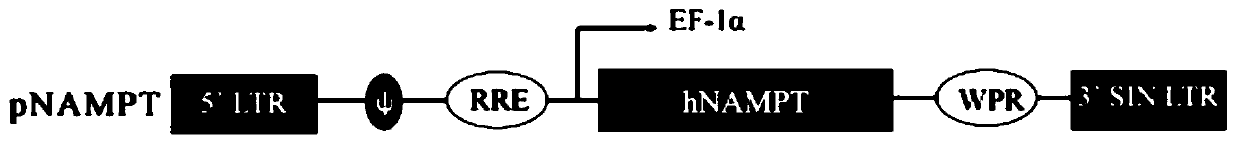
[0030] Chemically synthesize the hNAMPT gene, use the degeneracy of the gene code to replace certain bases, reduce the potential palindromic structure in the sequence to increase the titer of lentivirus production and expression in host cells, hNAMPT gene after base replacement The sequence is shown in SEQ ID NO:1. Then insert the hNAMPT gene sequence into the lentiviral vector pGIPZXbaI / MluI site to generate a new anti-aging lentiviral vector phNAMPT, as shown in the attached figure 1 shown. Finally, the gene sequence of the chemically synthesized and recombinant plasmid phNAMPT was analyzed by sequencing to be accurate.
[0031] SEQ ID NO: 1:
[0032]TCTAGACCTGTCCTCCGGCCCGAGATGAACCCCGCCGCCGAGGCCGAGTTCAACATCCTGCTGGCCACCGATAGCTACAAGGTGACCCACTACAAGCAGTACCCTCCCAACACCAGCAAGGTGTACAGCTACTTCGAGTGTCGCGAGAAGAAGACCGAGAACAGCAAGCTGAGGAAGGTGAAGTACGAAGAGACCGTGTTCTACGGCCTGCAGTACATCCTGAACAAGTACCTGAAGGGCAAGGTGGTGACCAAGGAGAA...
Embodiment 2
[0033] The packaging of embodiment 2NAMPT lentivirus
[0034] The lentiviral vector phNAMPT plasmid DNA and helper plasmid (pSPAX2.0 and pMD2G) DNA in Example 1 were co-transfected into 293T cells at an optimized ratio for lentiviral packaging. Among them, the pSPAX2.0 plasmid provides the reverse transcriptase necessary for lentiviral packaging, the Rev necessary for nucleoprotein and lentiviral RNA transport; the pMD2G plasmid provides the viral envelope VSV-G to ensure the broad host tropism of the lentivirus. In future tests, this pseudotropism will be further selected and optimized. This optimization can greatly improve the packaging efficiency of the lentivirus, ensuring that the phNAMPT lentivirus can infect skin cells or systemic tissue cells to the greatest extent when it is applied locally or systemically.
Embodiment 3
[0035] Purification of embodiment 3NAMPT lentivirus
[0036] After the 293T cells in Example 2 were cultured for 72 hours, the cell supernatant was collected and the titer of the lentivirus was measured, and the free host cell (293T cell) DNA / RNA was degraded by nuclease digestion, concentrated into a crude product by centrifugation, and used The titer of lentivirus was determined after resuspending the lentivirus particles in phosphate buffer solution; further purification was carried out by using ion exchange chromatography column, sucrose gradient centrifugation, etc. to remove impurity proteins and cell membrane fragments in the crude lentivirus product, and reach the clinical level for in vivo application. Then measure the lentivirus titer again, and calculate the lentivirus packaging efficiency and the yield of lentivirus in each concentration and purification step.
[0037] Among them, the ultracentrifugation method
[0038] 1. After sterilizing the Ultra-clearSW28 cen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com