Method for detecting related substances of flunarizine hydrochloride preparation
A technology of flunarizine hydrochloride and related substances, which is applied in the field of detection of related substances of flunarizine hydrochloride preparations, and can solve the problems of poor separation of impurities, small specifications, long detection time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 standard curve, limit of quantitation and rate of recovery
[0054] 1. Standard curve
[0055] Diluent: methanol: pH3.5 phosphate buffer = 75:25, v / v.
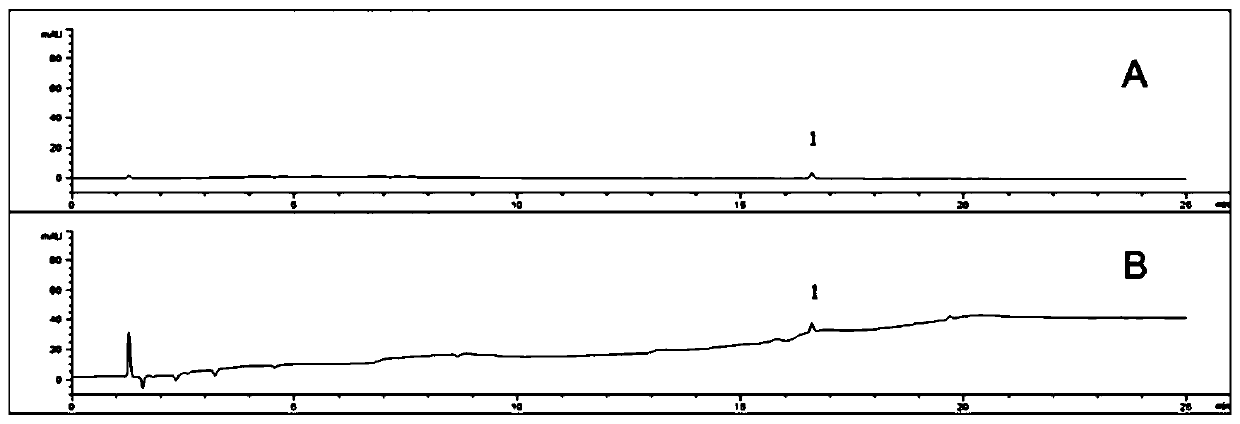
[0056] Accurately weigh 15 mg of impurity A, 12 mg of 4,4-difluorobenzhydryl alcohol, and 35.4 mg of flunarizine hydrochloride (equivalent to 30 mg of flunarizine), and place them in a 100ml measuring bottle, dissolve and dilute to the mark with methanol, and shake well , to prepare impurity A stock solution, 4,4-difluorobenzhydryl alcohol stock solution, and flunarizine hydrochloride stock solution respectively.
[0057] Accurately measure an appropriate amount of each of the above-mentioned stock solutions, and dilute them with a diluent into impurity A solution, 4,4-difluorobenzhydryl alcohol solution and flunarizine hydrochloride solution with different concentration gradients, and carry out high performance liquid chromatography detection (same as Embodiment 2), with the linear solution concentration...
Embodiment 2
[0085] 1. Instruments: High performance liquid chromatography, Agilent 1260 / 1100 high performance liquid chromatography, Chem Station chromatography workstation, Mettler FE20pH meter, Mettler ME204 / XS205 analytical balance.
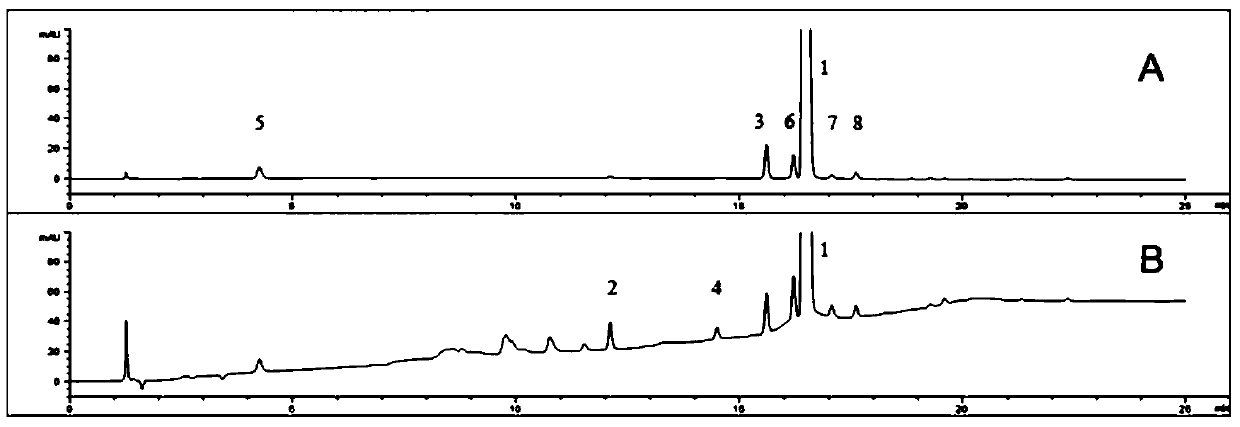
[0086] 2. Reagent: The test product is the contents of commercially available flunarizine hydrochloride capsules, and the standard product is flunarizine hydrochloride standard product; 7 impurity standard products: impurity A (1-[bis-(4-fluorophenyl ) methyl] piperazine), impurity B (1-[(4-fluorophenyl) phenylethyl]-4-[(2E)-3-phenylpropenyl] piperazine), impurity D (1-[ Bis-(4-fluorophenyl)methyl]-4-[(2Z)-3-phenylpropenyl]piperazine), trans-1-cinnamylpiperazine, 4,4-difluorobenzophenone , 4,4-difluorobenzhydryl alcohol, nitrogen oxides; methanol and acetonitrile are chromatographically pure, and the rest of the reagents are analytically pure.
[0087] Diluent: methanol: pH3.5 phosphate buffer = 75:25, v / v.
[0088] 3. Solution preparation
[0089] Tes...
Embodiment 3
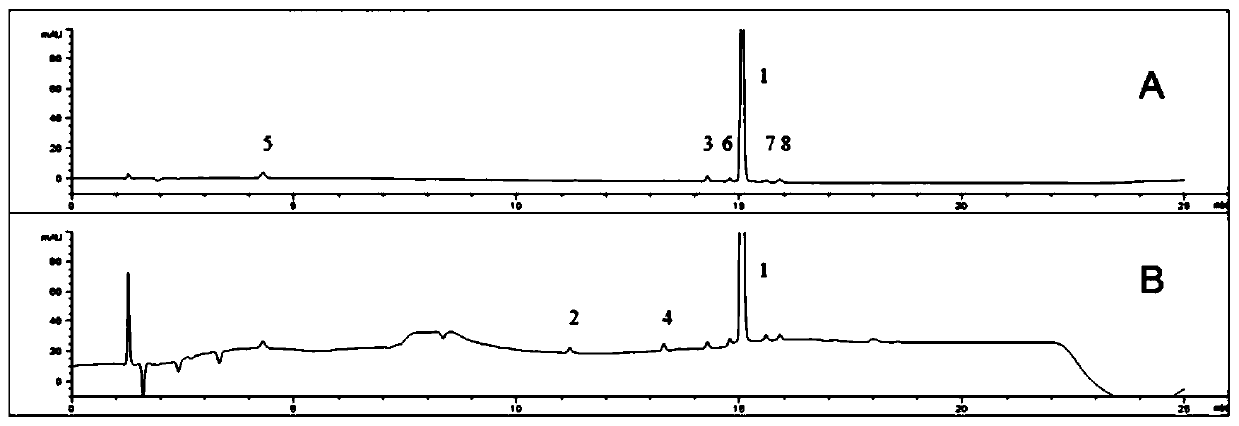
[0111] Need testing solution: get commercially available flunarizine hydrochloride tablet and grind finely, accurately weighed to be equivalent to flunarizine 30mg, place in 50mL volumetric flask, add diluent (same as embodiment 2), 40kHz ultrasonic 10min dissolves And dilute to the mark, filter with 0.45μm filter membrane, take the filtrate, as the test solution, the concentration of flunarizine is 0.6mg / ml.
[0112] The mixed solution of the test product and each impurity standard: Take commercially available flunarizine hydrochloride tablets and grind finely, accurately weighed to be equivalent to 30 mg of flunarizine, place it in a 50mL volumetric flask, and accurately measure each impurity standard for storage Liquid is appropriate in the same measuring bottle, then diluent (same as embodiment 2), 40kHz ultrasonic 10min dissolves and is diluted to scale, 0.45 μm membrane filtration, gets filtrate, as the solution of need testing sample and each impurity standard item, cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com