Electrochemical luminescence sensor system for determining adenosine triphosphate and preparation method and application thereof
An adenosine triphosphate, electrochemical technology, applied in the field of biomedical detection, can solve the problems of high cost, tedious time-consuming, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Au@Fe 3 o 4 - Preparation of substrate-aptazyme probes
[0095] (1) Fe 3 o 4 Preparation of nanoparticles
[0096] Weigh 0.65g FeCl 3 and 0.2g of trisodium citrate into a beaker filled with 20mL of ethylene glycol, stir well until it is completely dissolved, add 1.2g of sodium acetate into it, carry out magnetic stirring for 30min, add the above mixture into the reaction kettle, and react at 200°C 10h; the precipitate was washed with ethanol and water three times successively after magnetic separation to obtain Fe 3 o 4 Nanoparticles.
[0097] (2) Preparation of Au nanoparticles
[0098] Configure 50mL mass fraction as 0.01% HAuCl 4 Add the aqueous solution into a round-bottomed flask, heat it to boiling, add 1 mL of 0.1% trisodium citrate aqueous solution to the solution, continue heating and boiling for 15 min, and cool the sample to room temperature to obtain an Au nanoparticle solution.
[0099] (3)Au@Fe 3 o 4 Nanoparticle preparation
[0100] Weigh 10m...
Embodiment 2
[0104] RuSiO 2 -CS solution was prepared as follows:
[0105] Measure 2mL Triton X-100, 8mL cyclohexane, 2mL n-hexanol into the reaction vessel, mix well, add 350μL of 40mM Ru(bpy) to the mixture 3 Cl 2 Aqueous solution, after mixing, add 150μL TEOS and 100μL ammonia water, stir and react for 24h; add 5mL acetone, centrifuge, wash the precipitate with ethanol and water in turn, and resuspend the product in ethanol to obtain 4mg / mL RuSiO 2 solution.
[0106] Add 1mg CS to 1mL 2% acetic acid aqueous solution, ultrasonically disperse for 20min to obtain CS solution; take 1mL 4mg / mL RuSiO 2 Aqueous solution was added to 1mL CS solution, sonicated for 30min to obtain uniformly dispersed RuSiO 2 -CS solution.
Embodiment 3
[0108] Preparation method of electrochemiluminescent sensor for measuring Trigger DNA
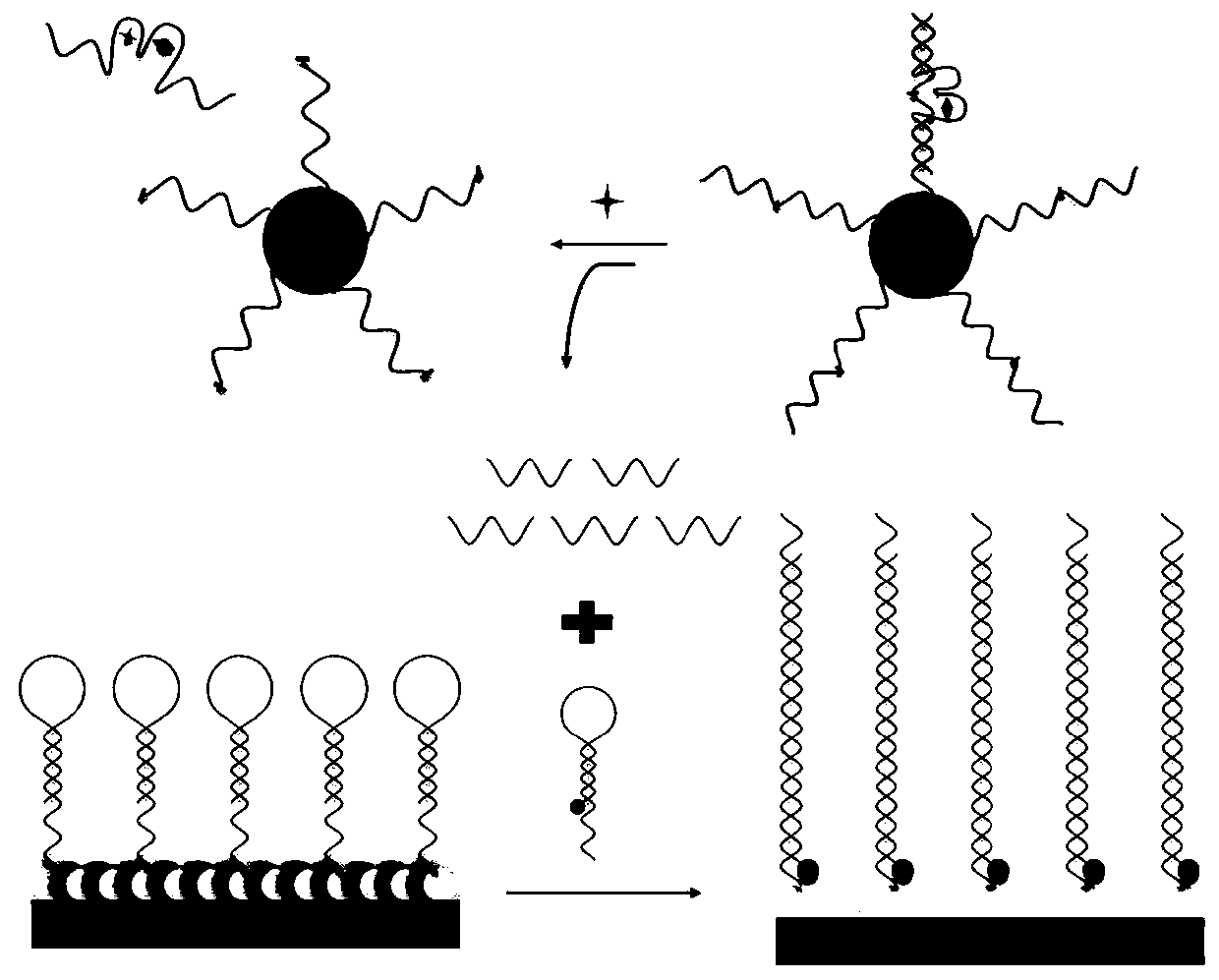
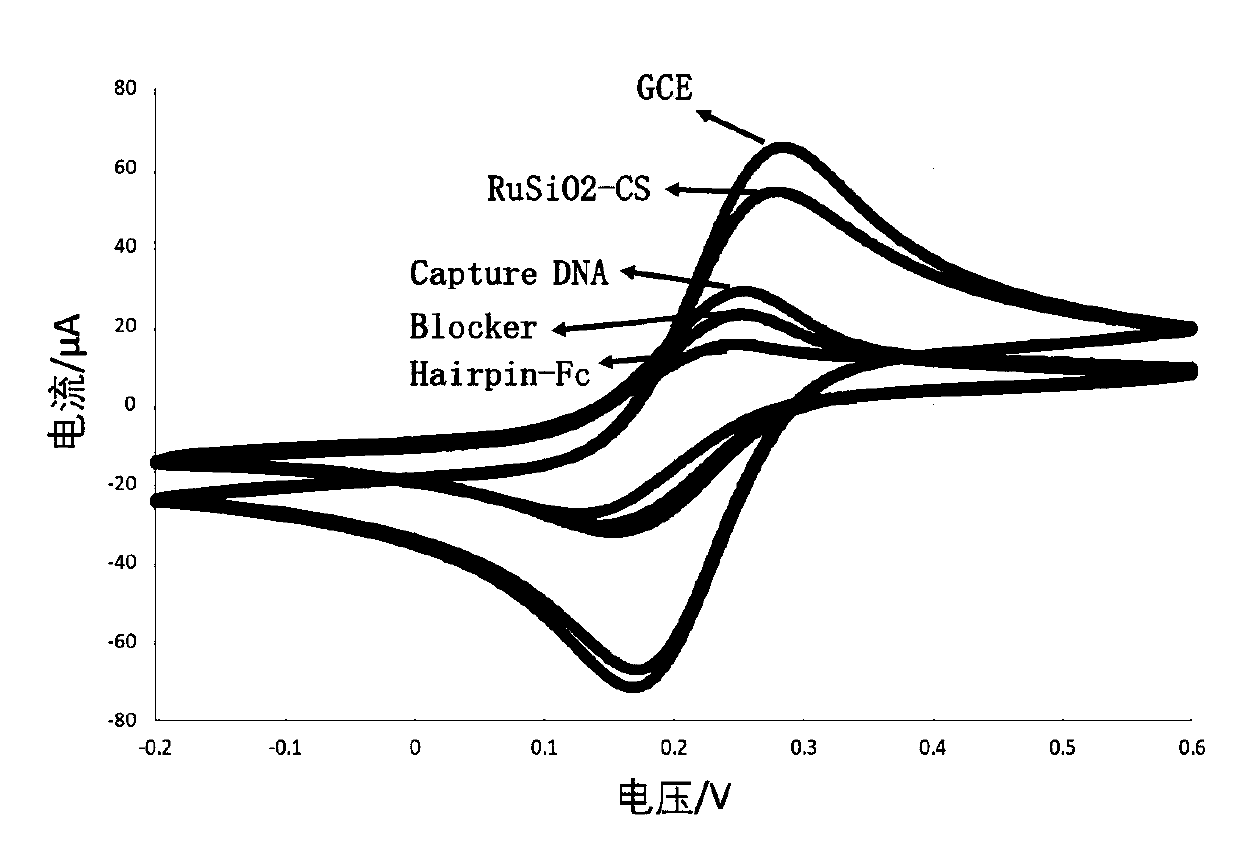
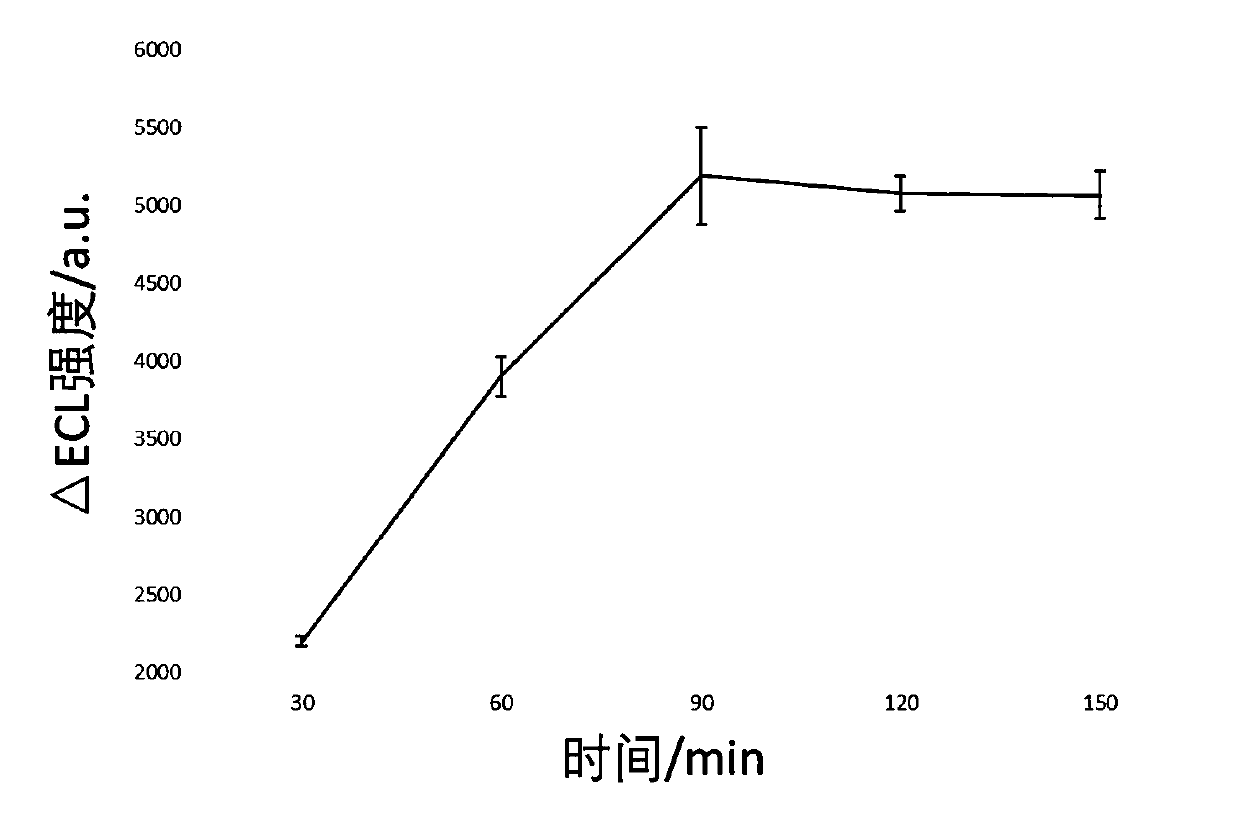
[0109] The preparation method of electrochemiluminescent sensor is as follows: figure 1 shown, including the following steps:
[0110] (1) Electrode pretreatment: use 0.3μm and 0.05μm Al in turn for the working electrode 2 o 3 After the powder is polished, ultrasonically clean it with water, ethanol, and water for 4 minutes;
[0111] (2) Modified RuSiO 2 : Take 10μL 2mg / mL RuSiO 2 - CS solution (Example 2) was added dropwise to the surface of the above-mentioned working electrode, left to dry at room temperature, washed with PBS solution, and dried in the air;
[0112] (3) Covalently link Capture DNA: dropwise add 2.5% glutaraldehyde aqueous solution to the surface of the above electrode, react at room temperature for 2 hours, wash with PBS solution, and dry in the air; add 10 μL of 4 μM Capture DNA dropwise to the electrode surface, 37 React at ℃ for 2 hours, wash with PBS solution, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com