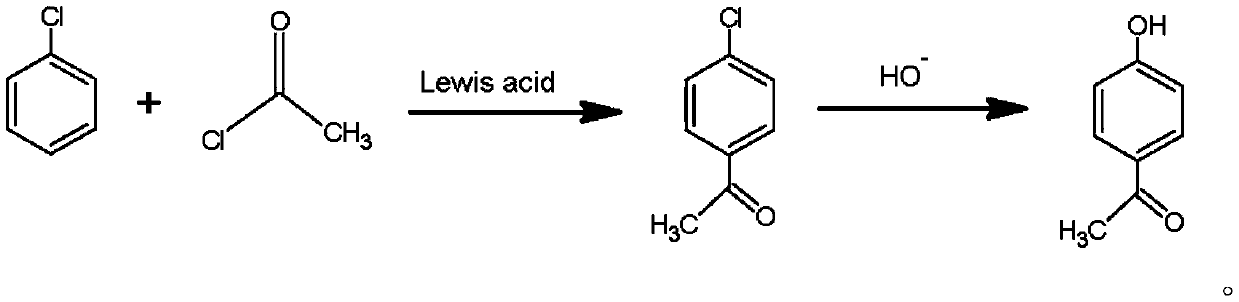
Preparation method of p-hydroxyacetophenone
A technology of p-hydroxyacetophenone and chlorobenzene, which is applied in the field of preparation of p-hydroxyacetophenone, can solve the problems of unfavorable industrial application, complicated preparation method, and many by-products, achieve good production and application prospects, increase production volume, The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take a 5L three-necked bottle, weigh 1120g chlorobenzene, 1000g acetyl chloride and 10g aluminum chloride respectively, put the chlorobenzene and aluminum chloride into the three-necked bottle, and place it in a freezing tank, control the temperature below 10°C, and put 1000g Acetyl chloride was slowly added dropwise to the three-necked flask, and the dropping time was 3 hours. After the dropwise addition, the three-necked bottle was placed in a water bath, heated to 60°C, and kept for 2 hours. After the insulation was completed, the reaction system was filtered to obtain the filtrate. Alkali was configured into 10% sodium hydroxide aqueous solution, mixed sodium hydroxide aqueous solution and filtrate, transferred to a 10L three-necked flask, heated to 120°C in an oil bath, and refluxed for 4 hours. After the reaction was completed, hydrochloric acid was added dropwise to adjust the pH to 3.12. Stir and stand for stratification, take the organic layer, heat up to 125°C,...
Embodiment 2
[0028] Take a 5L three-neck bottle, weigh 1120g chlorobenzene, 1200g acetyl chloride and 20g zinc chloride respectively, put the chlorobenzene and zinc chloride into the three-necked bottle, and place them in a freezing tank, control the temperature below 10°C, and put 1200g Acetyl chloride was slowly added dropwise to the three-necked flask, and the dropping time was 3.5 hours. After the dropwise addition, the three-necked bottle was placed in a water bath, and the temperature was raised to 80°C, and the reaction was kept for 3 hours. The caustic soda is configured into a 20% sodium hydroxide aqueous solution, and the sodium hydroxide aqueous solution is mixed with the filtrate, transferred to a 10L three-necked bottle, heated to 150°C in an oil bath, and refluxed for 8 hours. After the reaction is completed, add hydrochloric acid dropwise to adjust the pH to 5.05 , stir and stand for stratification, take the organic layer, heat up to 130°C, distill, until there is no obvious ...
Embodiment 3
[0030] Take a 5L three-necked bottle, weigh 1120g chlorobenzene, 1100g acetyl chloride and 15g ferric chloride respectively, put chlorobenzene and ferric chloride into the three-necked bottle, and place them in a freezing tank, control the temperature below 10°C, and put 1100g Acetyl chloride was slowly added dropwise to the three-necked flask, and the dropping time was 3 hours. After the dropwise addition, the three-necked bottle was placed in a water bath, and the temperature was raised to 70°C, and the reaction was kept for 2.5 hours. The caustic soda is configured into a 15% sodium hydroxide aqueous solution, and the sodium hydroxide aqueous solution and the filtrate are mixed, transferred to a 10L three-necked bottle, heated to 130°C in an oil bath, and refluxed for 6 hours. After the reaction is completed, add hydrochloric acid dropwise to adjust the pH to 4.26 , stirring and standing for stratification, take the organic layer, heat up to 128°C, distill, until there is no...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com