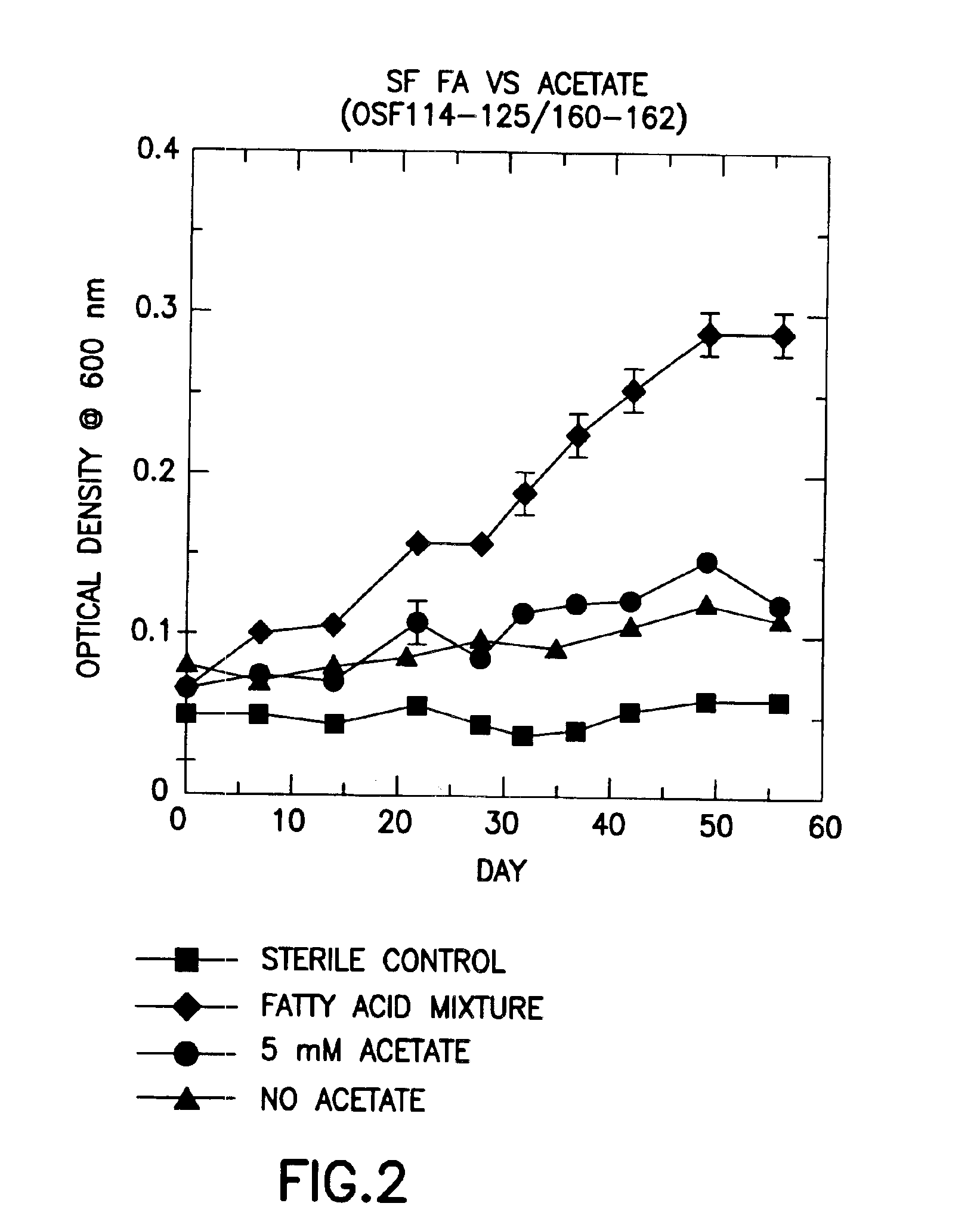
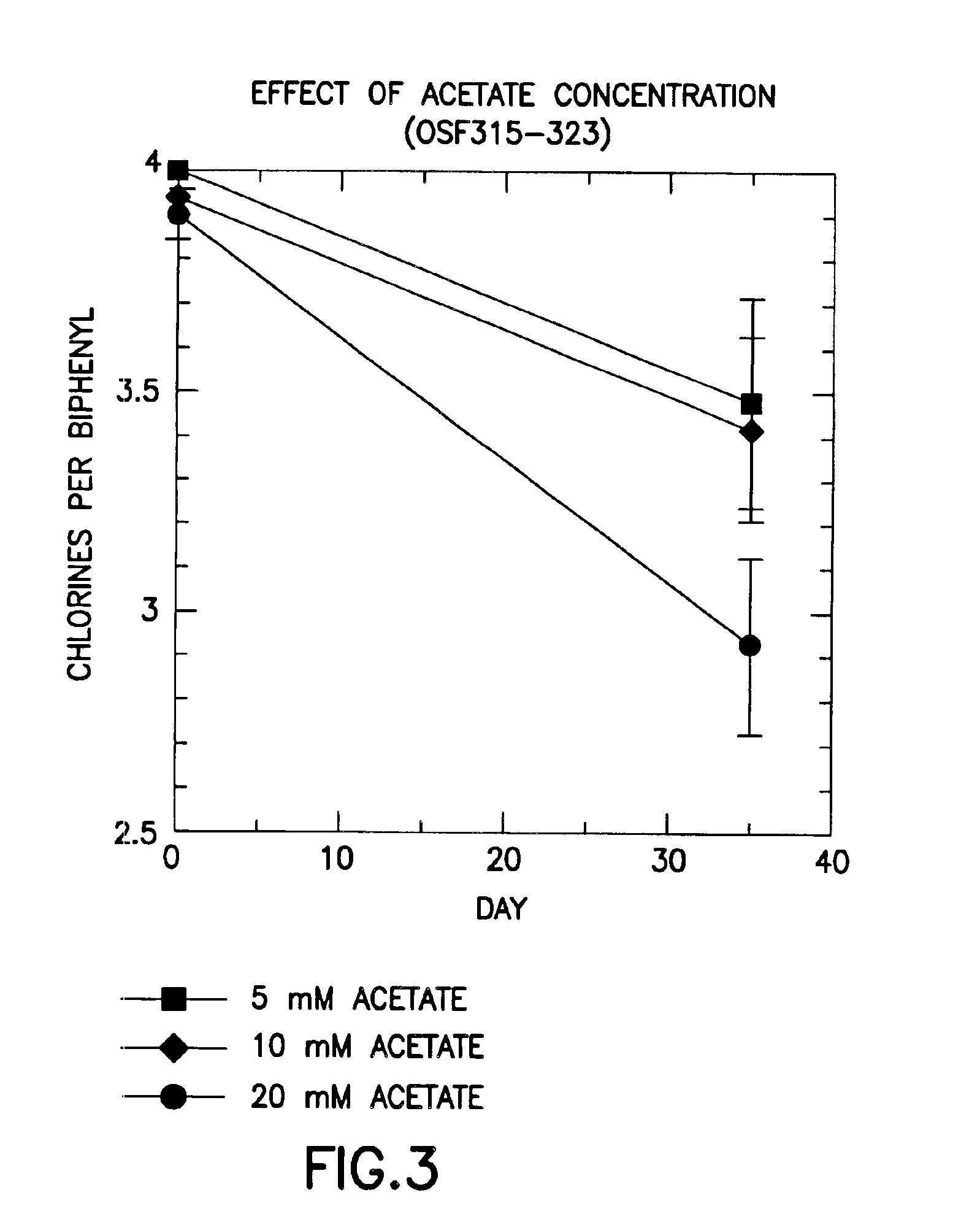
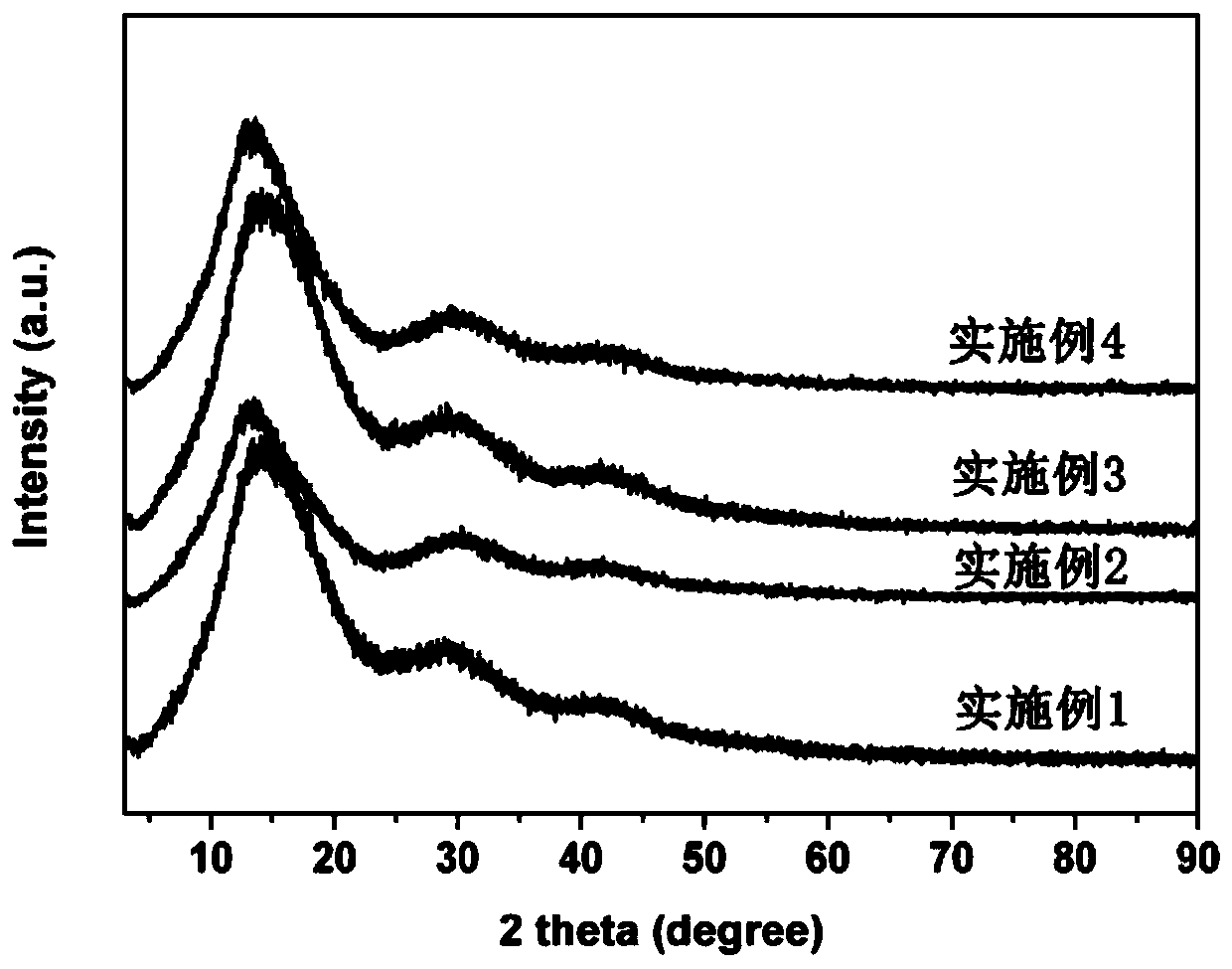
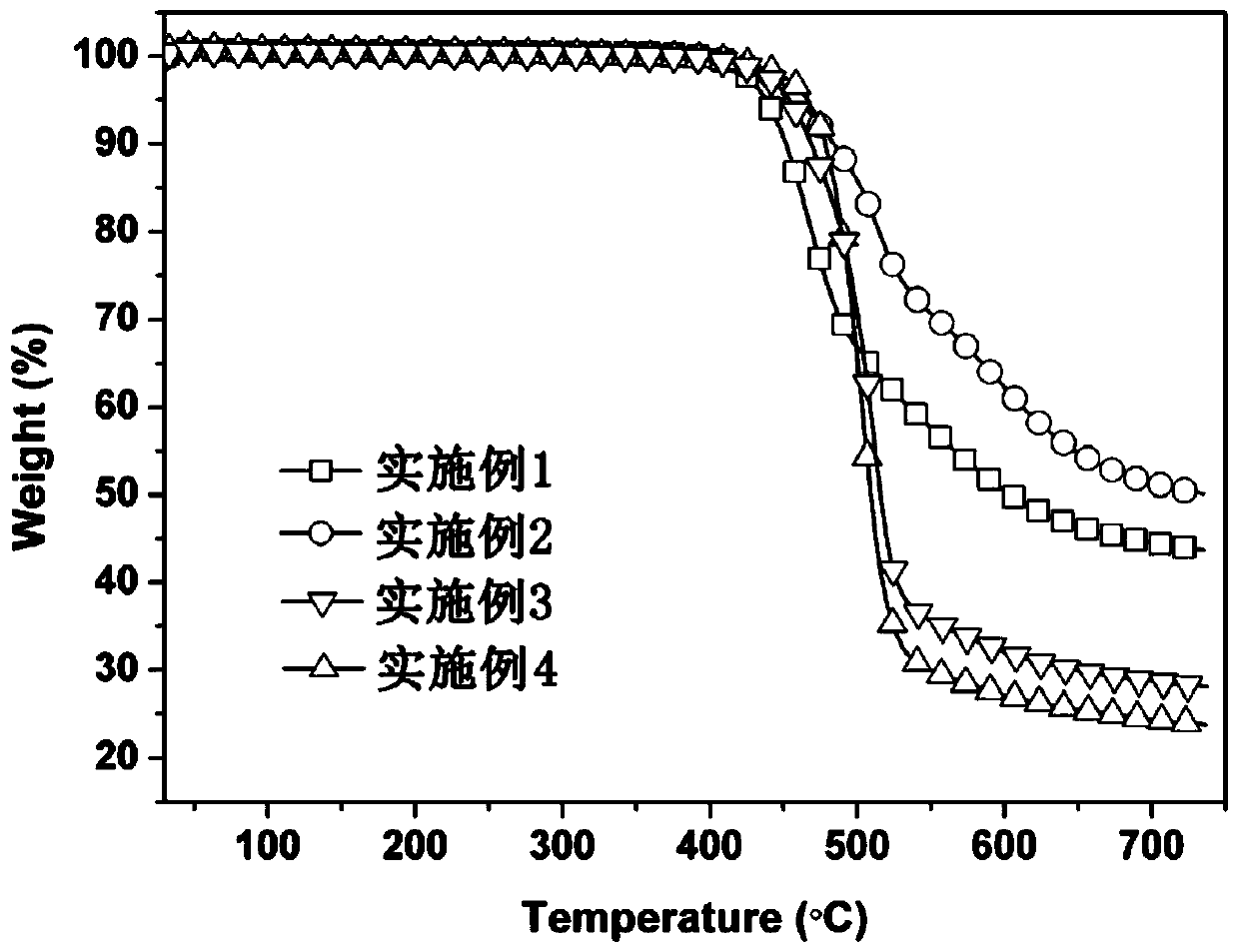
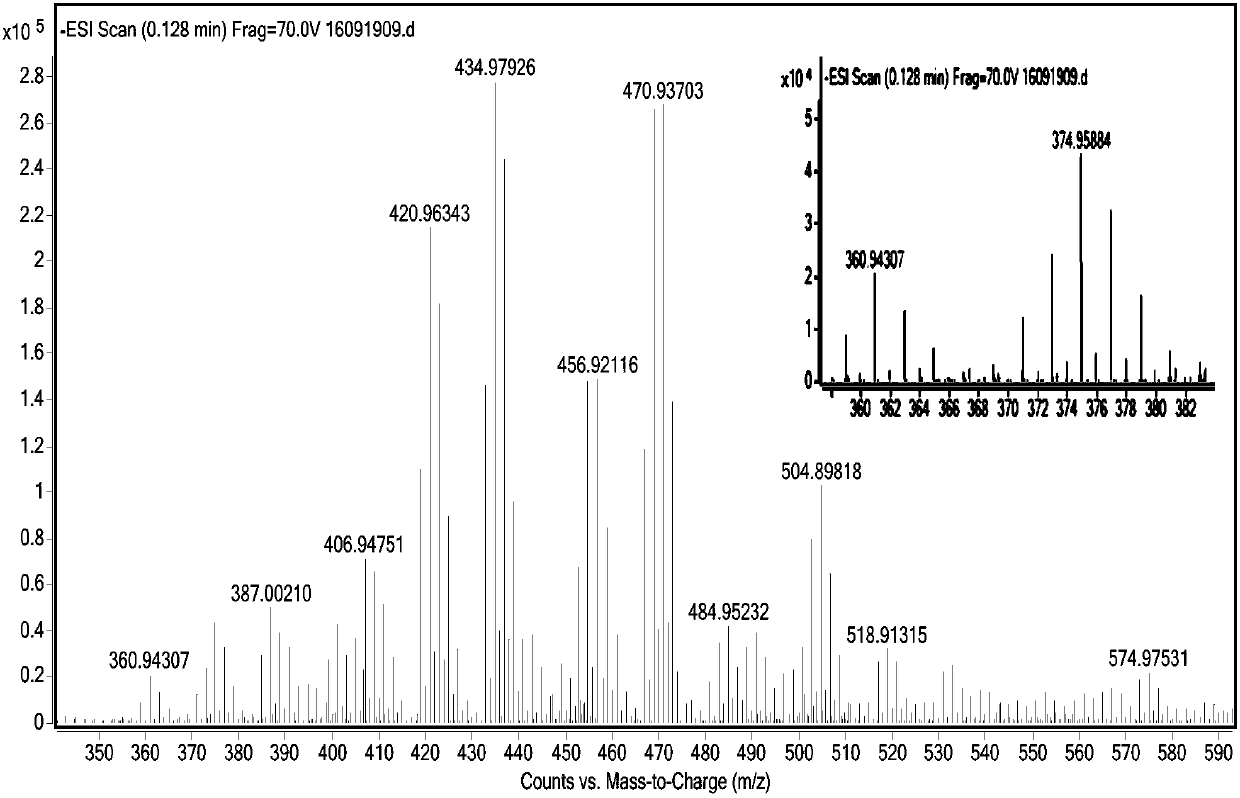
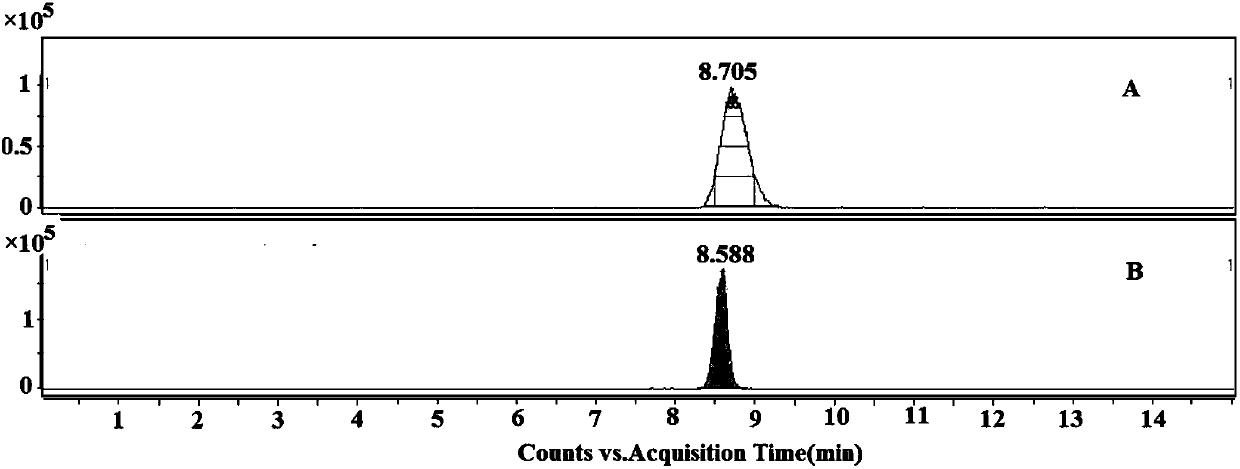
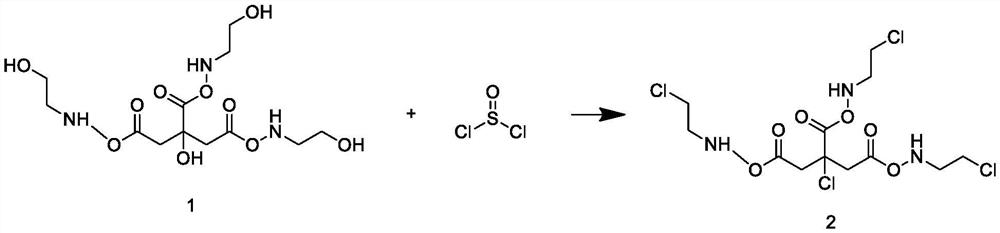
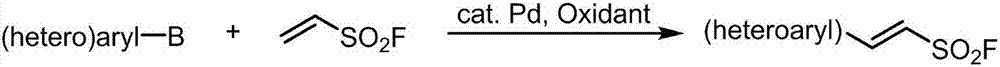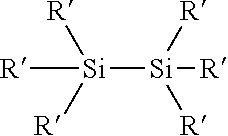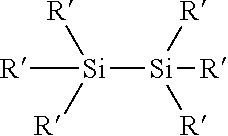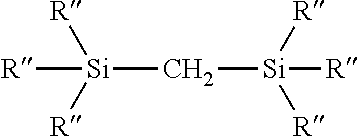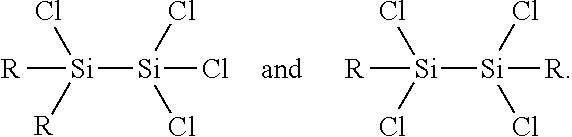Patents
Literature
31 results about "Chlorine substituent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Functionalized Copolymers of Terminally Functionalized Perfluoro (Alkyl Vinyl Ether) Reactor Wall for Photochemical Reactions, Process for Increasing Fluorine Content in Hydrocaebons and Halohydrocarbons and Olefin Production
InactiveUS20070265368A1Preparation by hydrogen halide split-offCatalyst activation/preparationVinyl etherHalohydrocarbon
A photochemical reaction apparatus including a reactor and a light source situated so that light from the light source is directed through a portion of the reactor wall is disclosed. The apparatus is characterized by the portion of the reaction wall comprising a functionalized copolymer of a terminally functionalized perfluoro(alkyl vinyl ether). Also described is a photochemical reaction process using said reactor. The functional group of the copolymer of the apparatus and the process is selected from —SO2F, —SO2CI, —SO3H, —CO2R (where R is H or C1-C3 alkyl), —PO3H2, and salts thereof. A process for increasing the flourine content of at least one compound selected from hydrocarbons and halohydrocarbons, comprising: (a) photochlorinating said at least one compound, and (b) reacting the halogenated hydrocarbon in (a) with HF. A process for producing an olefinic compound, comprising: (a) photochlorinating at least one compound selected from hydrocarbons and halohydrocarbons containing at least two carbon atoms and at least two hydrogen atoms to produce a halogenated hydrocarbon containing a hydrogen substituent and a chlorine substituent on adjacent carbon atoms; and (b) subjecting the halogenated hydrocarbon produced in (a) to dehydrohalogenation.
Owner:EI DU PONT DE NEMOURS & CO
Use of copolymers of perfluoro(alkyl vinyl ether) for photochemical reactions
InactiveUS7943015B2Impression capsPreparation by hydrogen halide split-offVinyl etherHalohydrocarbon
A photochemical reaction apparatus including a reactor and a light source situated so that light from the light source is directed through a portion of the reactor wall is disclosed. The apparatus is characterized by the portion of the reaction wall comprising a copolymer of a perfluoro (alkyl vinyl ether). The perfluoro (alkyl vinyl ether) is selected from the group consisting of CF30CF═CF2, C2F5OCF═CF2, C3F7OCF═F2, and mixture thereof. Also disclosed is a photochemical reaction process wherein light from a light source is directed through said reactor wall to interact with reactants in said reactor. A process for increasing the fluorine content of at least one compound selected from hydrocarbons and halohydrocarbons, comprising: (a) photochlorinating said at least one compound; and (b) reacting the halogenated hydrocarbon produced in (a) with HF. A process for producing an olefinic compound, comprising: (a) photochlorinating at least one compound selected from hydrocarbons and halohydrocarbons containing at least two carbon atoms and at least two hydrogen atoms to produce a halogenated hydrocarbon containing a hydrogen substituent and a chlorine substituent on adjacent carbon atoms; and (b) subjecting the halogenated hydrocarbon produced in (a) to dehydrohalogenation.
Owner:EI DU PONT DE NEMOURS & CO
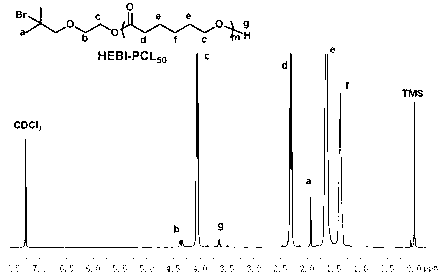
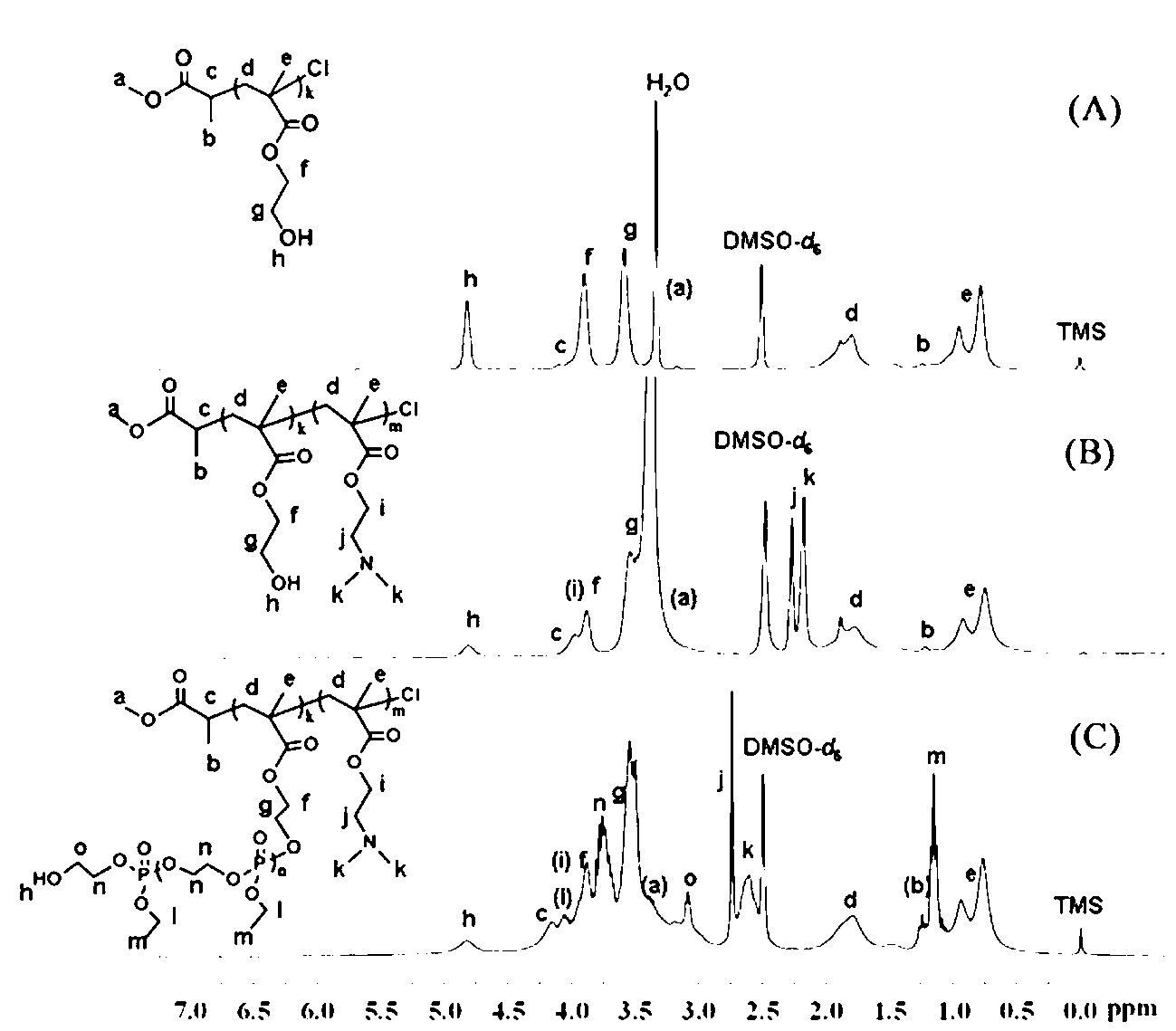
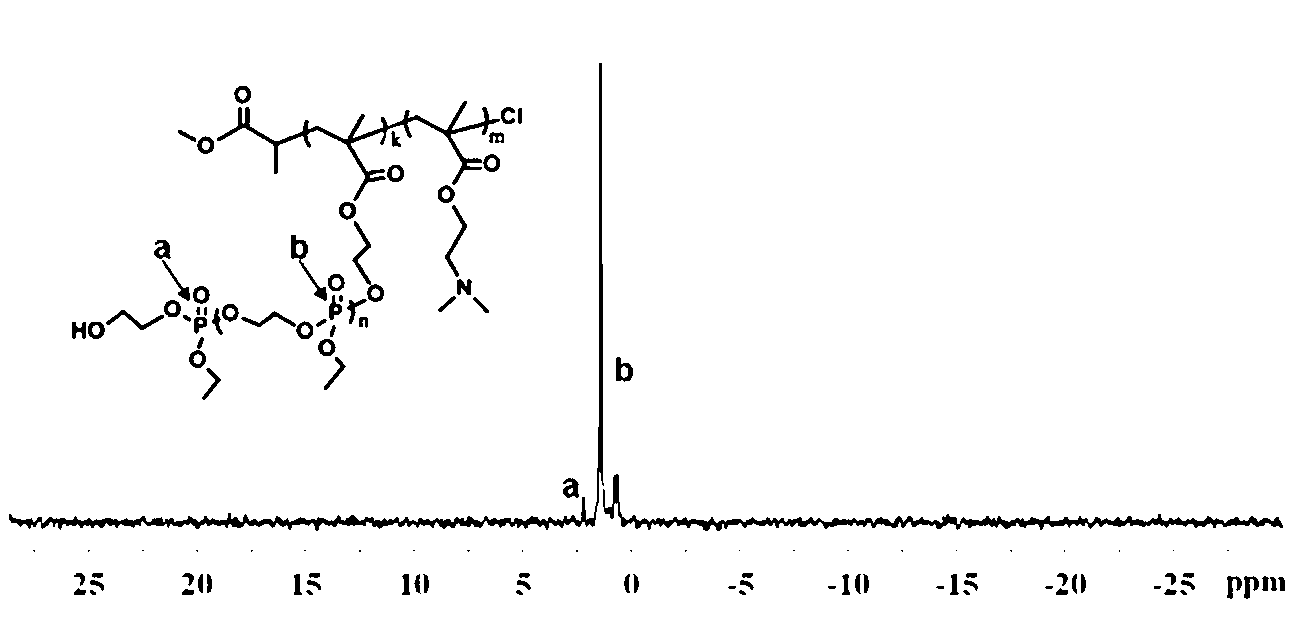
Cationic triblock copolymer based on biodegradable polyphosphate ester and application thereof
InactiveCN102796236APromote degradationGood biocompatibilityOther foreign material introduction processesPhosphateRing-opening polymerization
The invention discloses a preparation method of a cationic triblock copolymer based on biodegradable polyphosphate ester and application thereof in medicine carriers and gene vectors. Caprolactone is subjected to ring-opening polymerization by a micro-molecule initiator containing halogen on one terminal and hydroxyl group on the other terminal under the catalytic action of stannous octoate, so as to obtain a macro-molecule ATRP initiator containing hydroxyl group on one terminal and bromine or chlorine substituent on the other terminal, and then an annular phosphate ester monomer is subjected to ring-open polymerization, so as to obtain a diblock copolymer of the poly-caprolactone and poly-phosphate ester; and finally the diblock copolymer is subjected to solution ATRP reaction with pH sensitive nitrogen-contained monomers under the action of an ATRP catalyst and a ligand, so as to obtain a pH sensitive triblock copolymer based on poly-phosphate ester, which can be used as hydrophobic medicine carrier and gene vector.
Owner:SUZHOU UNIV
Selectively reacting olefins having a terminal CF2 group in a mixture
ActiveUS7728183B2Halogenated hydrocarbon separation/purificationSulfuric acid esters preparationChlorine substituentAlkoxy group
A process is disclosed for reducing the mole ratio of (1) compounds of the formula Y1Y2C═CF2 wherein Y1 and Y2 are each independently H, F, Cl, Br, C1-C6 alkyl or C1-C6 haloalkyl containing no more than 3 chlorine substituents, 2 bromine substituents and 1 iodo substituent to (2) saturated compounds of the formula CdHeFfClgBrhIk wherein d is an integer from 1 to 10, and e+f+g+h+k is equal to 2d+2, provided that g is 0, 1, 2 or 3, h is 0, 1 or 2 and k is 0 or 1 and / or unsaturated compounds of the formula Y3Y4C═CY5Y6, wherein Y3, Y5 and Y6 are each independently H, F, Cl, Br, C1-C6 alkyl or C1-C6 haloalkyl containing no more than 3 chlorine substituents, 2 bromine substituents and 1 iodo substituent, provided that Y5 and Y6 are not both F, and Y4 is C1-C6 alkyl or C1-C6 haloalkyl containing no more than 3 chlorine substituents, 2 bromine substituents and 1 iodo substituent, in a mixture. The process involves contacting the mixture with at least one selective removal agent selected from the group consisting of SO3 and RSO3H, wherein R is selected from the group consisting of F, Cl, OH, C1-C8 alkyl, C1-C8 fluoroalkyl, and C1-C8 fluoroalkoxyalkyl containing no more than two ether oxygens to selectively react the formula Y1Y2C═CF2 compounds.
Owner:HONEYWELL INT INC
Compositions and methods for microbial dechlorination of polychlorinated biphenyl compounds
InactiveUS6946248B2Maximize overall degradationFacilitates bioremediation treatmentBacteriaSolid waste disposalMicroorganismPolychlorinated biphenyl
Bioremediative microorganisms comprising a 16S ribosomal subunit nucleic acid sequence and useful in various methods for dechlorinating chlorinated biphenyls (PCBs), including anaerobic dechlorination of ortho- and double-flanked chloro substituents of PCBs. The methods of bioremediation may employ consortia of microbially effective species, e.g., aerobic as well as anaerobic species, to dechlorinate corresponding PCB mixtures containing widely varying and significant numbers of PCB congeners.
Owner:NIVERSITY OF MARYLAND BALTIMORE COUNTY +1
Selectively Reacting Olefins Having a Terminal CF2 Group in A Mixture
ActiveUS20090012336A1Reducing mole ratioHalogenated hydrocarbon separation/purificationSulfuric acid esters preparationChlorine substituentBromine
A process is disclosed for reducing the mole ratio of (1) compounds of the formula Y1Y2C═CF2 wherein Y1 and Y2 are each independently H, F, Cl, Br, C1-C6 alkyl or C1-C6 haloalkyl containing no more than 3 chlorine substituents, 2 bromine substituents and 1 iodo substituent to (2) saturated compounds of the formula CdHeFfClgBrhIk wherein d is an integer from 1 to 10, and e+f+g+h+k is equal to 2d+2, provided that g is 0, 1, 2 or 3, h is 0, 1 or 2 and k is 0 or 1 and / or unsaturated compounds of the formula Y3Y4C═CY5Y6, wherein Y3, Y5 and Y6 are each independently H, F, Cl, Br, C1-C6 alkyl or C1-C6 haloalkyl containing no more than 3 chlorine substituents, 2 bromine substituents and 1 iodo substituent, provided that Y5 and Y6 are not both F, and Y4 is C1-C6 alkyl or C1-C6 haloalkyl containing no more than 3 chlorine substituents, 2 bromine substituents and 1 iodo substituent, in a mixture. The process involves contacting the mixture with at least one selective removal agent selected from the group consisting of SO3 and RSO3H, wherein R is selected from the group consisting of F, Cl, OH, C1-C8 alkyl, C1-C8 fluoroalkyl, and C1-C8 fluoroalkoxyalkyl containing no more than two ether oxygens to selectively react the formula Y1Y2C═CF2 compounds.
Owner:HONEYWELL INT INC
Chlorinated 2, 4, 6-trinitro-1, 3- distyryl benzene derivatives as well as preparation method and application thereof
InactiveCN104016868AEasy to passAvoid decompositionOrganic chemistryOrganic compound preparationChemical structureOrtho position
The invention discloses 2, 4, 6-trinitro-1, 3-distyryl benzene derivatives containing chlorine substituent as well as a preparation method and an application thereof. The chemical structure of the compounds is characterized in that three nitryls exist at positions 2, 4, 6 of a middle benzene ring of 1, 3-distyryl benzene, and ortho-positions, meta-positions and para-positions of benzene rings at the two sides are connected to chlorine atoms, or the ortho-positions and the para-positions are simultaneously connected to the chlorine atoms. The chemical structure of the compounds is confirmed to be the trans-structure when the coupling constant of diphenylethene double bonds in a nuclear magnetic resonance spectrum is about 16.0Hz. The 2, 4, 6-trinitro-1, 3-distyryl benzene derivatives containing chlorine substituent are synthesized through microwave assistance under a catalysis condition, are simple in a preparation technology, solvent-free, fast, green and safe and can be potentially applied to the field of luminescent devices and energetic materials.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of 2-aryl-ethenylsulfonyl chloride compound
InactiveCN107188834AReduce loadLow equipment requirementsSulfonic acid preparationPalladium catalystEthyl Chloride
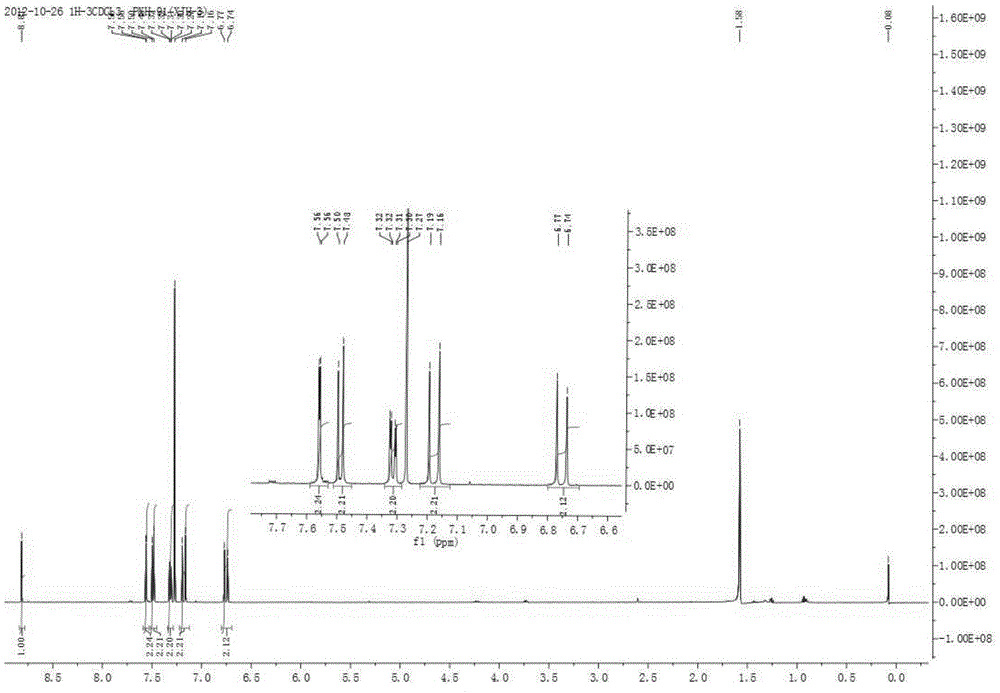
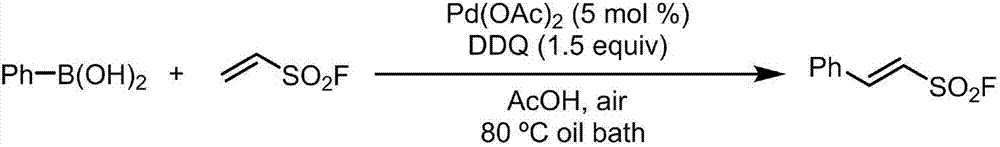
The invention discloses a preparation method of 2-aryl-ethenylsulfonyl chloride compound, comprising the steps of using arylboronic acid, arylboronic acid ester or arylboronic acid salt as a raw material, mixing with ESF (ethenylsulfonyl chloride), palladium catalyst, an oxidant and a solvent, allowing reacting at 20-120 DEG C for 2 h and longer, separating and purifying to obtain an ethenylsulfonyl chloride product. A molar ratio of arylboronic acid (ester) and its salt, ethenylsulfonyl chloride, the palladium catalyst and the oxidant is 1:(1-10):(0.000001-0.2):(0.5-5). The preparation method has the advantages that raw materials are simple and easy to obtain, large-scale commercial supply is available for both arylboronic acid (ester) and its salt, the arylboronic acid (ester) and its salt are tolerant to most functional groups and good in reaction selectivity (both bromine and chlorine substituent on aryl are not involved in the reaction under same reaction conditions), and the preparation method has good technical potential in large-scale preparation in labs and industrial amplified production.
Owner:WUHAN UNIV OF TECH
Method of preparing arylamine chloride by high selective catalytic hydrogenation of chlorine substituent aromatic nitro compound
InactiveCN1919832AHigh puritySimple production processOrganic compound preparationAmino compound preparationNitro compoundChlorine substituent
The invention discloses a load-typed nanometer ruthenium / carbon catalyst, which is characterized by the following: hydrogening chloride aromatic compound based on high selective catalyst under mild condition to prepare arylamine chloride without antichlorination reaction; improving receiving rate by over 99.9%.
Owner:DALIAN UNIV OF TECH
Colorless transparent non-fluorine polyimide film with low thermal expansion coefficient and preparation method and application thereof
The invention discloses a preparation method and application of a colorless transparent non-fluorine polyimide film with a low thermal expansion coefficient. The film is prepared from an alicyclic dianhydride monomer and an aromatic diamine monomer containing a rigid amide bond and an alkyl or chlorine substituent through a high-temperature solution polycondensation method. The alicyclic dianhydride monomer can be hydrogenated pyromellitic dianhydride (HPMDA) or hydrogenated biphenyl tetracarboxylic dianhydride (HBPDA); the aromatic diamine monomer containing the rigid amide bond and the alkylor chlorine substituent can be selected from 2-methyl-4, 4'-diaminobenzanilide, 2-chloro-4, 4'-diaminobenzanilide, 2, 3'-dimethyl-4, 4'-diaminobenzanilide and the like. According to the invention, the coefficient of thermal expansion (CTE) of the film can be significantly reduced on the premise of maintaining good solubility of the PI resin and good optical transparency of the PI film. And the CTE value can be further reduced after the film is compounded with the silicon dioxide colloid.
Owner:威海新元科盛新材料有限公司
Use of Copolymers of Perfluoro(Alkyl Vinyl Ether) for Photochemical Reactions
InactiveUS20080015277A1Impression capsPreparation by hydrogen halide split-offVinyl etherHalohydrocarbon
A photochemical reaction apparatus including a reactor and a light source situated so that light from the light source is directed through a portion of the reactor wall is disclosed. The apparatus is characterized by the portion of the reaction wall comprising a copolymer of a perfluoro (alkyl vinyl ether). The perfluoro (alkyl vinyl ether) is selected from the group consisting of CF30CF═CF2, C2F5OCF═CF2, C3F7OCF═F2, and mixture thereof. Also disclosed is a photochemical reaction process wherein light from a light source is directed through said reactor wall to interact with reactants in said reactor. A process for increasing the fluorine content of at least one compound selected from hydrocarbons and halohydrocarbons, comprising: (a) photochlorinating said at least one compound; and (b) reacting the halogenated hydrocarbon produced in (a) with HF. A process for producing an olefinic compound, comprising: (a) photochlorinating at least one compound selected from hydrocarbons and halohydrocarbons containing at least two carbon atoms and at least two hydrogen atoms to produce a halogenated hydrocarbon containing a hydrogen substituent and a chlorine substituent on adjacent carbon atoms; and (b) subjecting the halogenated hydrocarbon produced in (a) to dehydrohalogenation.
Owner:EI DU PONT DE NEMOURS & CO
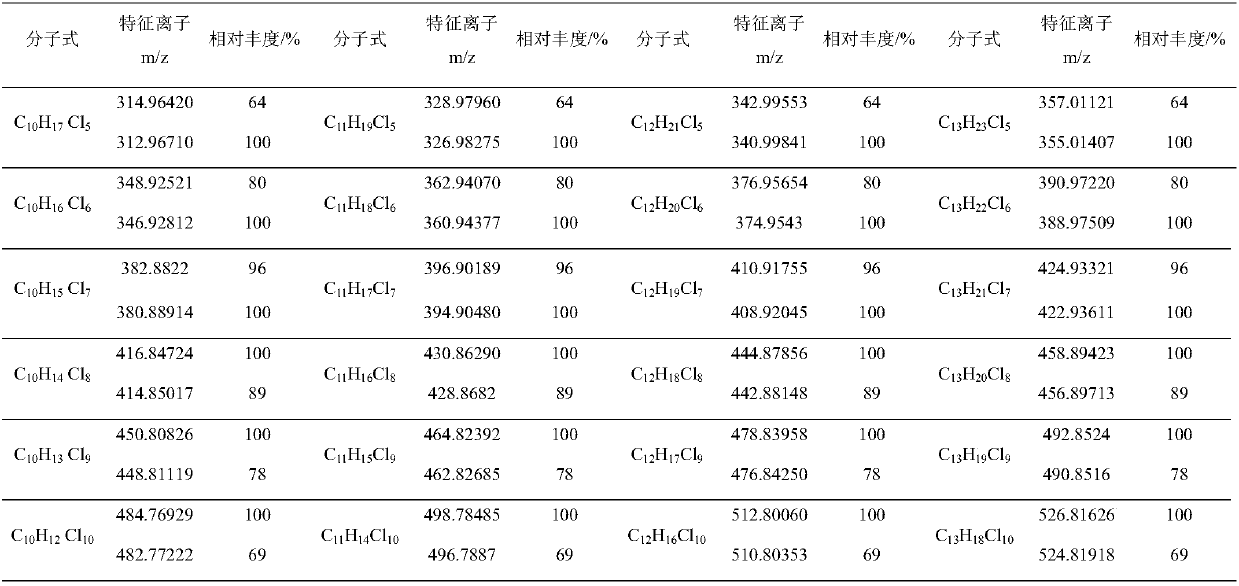
Measurement method of content of short-chain chlorinated paraffin
The invention discloses a measurement method of the content of short-chain chlorinated paraffin. According to the method, an ultra-performance liquid chromatography-serially-connected quadrupole time-of-flight mass spectrometry instrument is adopted for measuring a chlorinated paraffin sample and a short-chain chlorinated paraffin standard substance, and an anion full-scanning mode is adopted formeasuring mass spectrometric data of 24 compounds in the short-chain chlorinated paraffin in which the carbon number is 10-13 and the number of chlorine substituents is 5-10; two isotope ion peaks high in abundance and accurate in mass are selected from each molecular formula to serve as feature ions, 48 isotope feature ions accurate in mass number are extracted from a total ion flow chromatogram,with the abundance of two feature ion peaks in each kind of molecular formula short-chain chlorinated paraffin compounds as qualitative reference, the total peak area of the 48 isotope feature ions is adopted for quantification, a quantification result is contrasted with the short-chain chlorinated paraffin standard substance at last, and the content of the short-chain chlorinated paraffin is calculated. The measurement method solves the problems that the measurement of the short-chain chlorinated paraffin is interfered by medium-chain chlorinated paraffin, and the short-chain chlorinated paraffin is difficult to separate from the medium-chain chlorinated paraffin; the method has the advantages of being accurate and reliable in result and high in sensitivity.
Owner:INST OF ANALYSIS GUANGDONG ACAD OF SCI (CHINA NAT ANALYTICAL
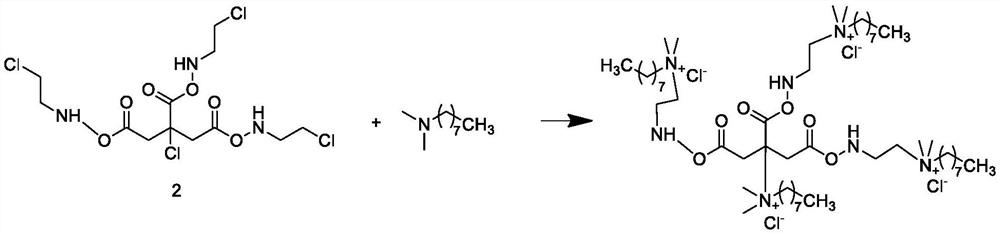
Synthesis method and application of 2-chloride, 3-poly hydroxypropyl dodecylamine quaternary ammonium salt surfactant
InactiveCN106422962ALow priceImprove conversion rateTransportation and packagingMixingChlorine substituentSynthesis methods
The invention discloses a synthesis method and an application of a 2-chloride, 3-poly hydroxypropyl dodecylamine quaternary ammonium salt surfactant. According to the synthesis method of the surfactant, firstly, dodecylamine and epichlorohydrin react to synthesize an intermediate with a di-chlorine substituent group, and the intermediate and N,N-dimethyl dodecylamine react to synthesize the 2-chloride, 3-poly hydroxypropyl dodecylamine quaternary ammonium salt surfactant. The method realizes synthesis of a target product with a two-step method and has the advantages that the cost is low and the surface activity of the product is high. The synthesized 2-chloride, 3-poly hydroxypropyl dodecylamine quaternary ammonium salt surfactant is applied to a combinational surfactant, and the performance of an obtained surfactant with a combined system is superior to that of a corresponding single system.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Process for purification of chlorinated alkenes
ActiveCN101903315APreparation by dehalogenationPreparation by hydrogen halide split-offChlorine substituentAlkene
Purified chlorinated alkenes are produced by a process in which a mixture of i) a first chlorinated alkene that has at least one beta-chlorine substituent and no alpha-chlorine substituents and ii) a second chlorinated alkene that has at least one alpha-chlorine substituent is contacted with chlorine in an amount sufficient to further chlorinate the second chlorinated alkene, but which is insufficient to cause conversion of more than 20% of the first chlorinated alkene. The resultant reaction product may be easily enriched to provide a chlorinated alkene product wherein a) the weight percentage of chlorinated alkenes having at least one beta-chlorine substituent and no alpha-chlorine substituents, based on the total weight of the chlorinated alkenes present in the enriched chlorinated alkene product compared to b) the weight percentage of chlorinated alkenes having at least one beta-chlorine substituent and no alpha-chlorine substituents, based on the total weight of the chlorinated alkenes present in the mixture prior to chlorination is increased by at least 0.25 wt.%.
Owner:DENKA PERFORMANCE ELASTOMER LLC (N D GES DES STAATES DELAWARE)
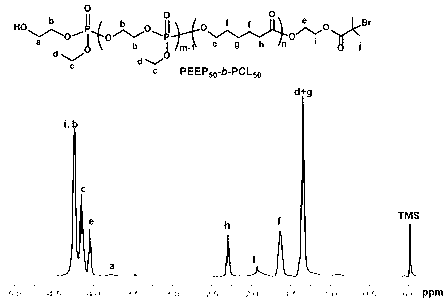
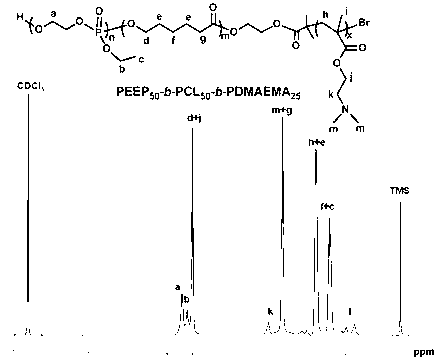
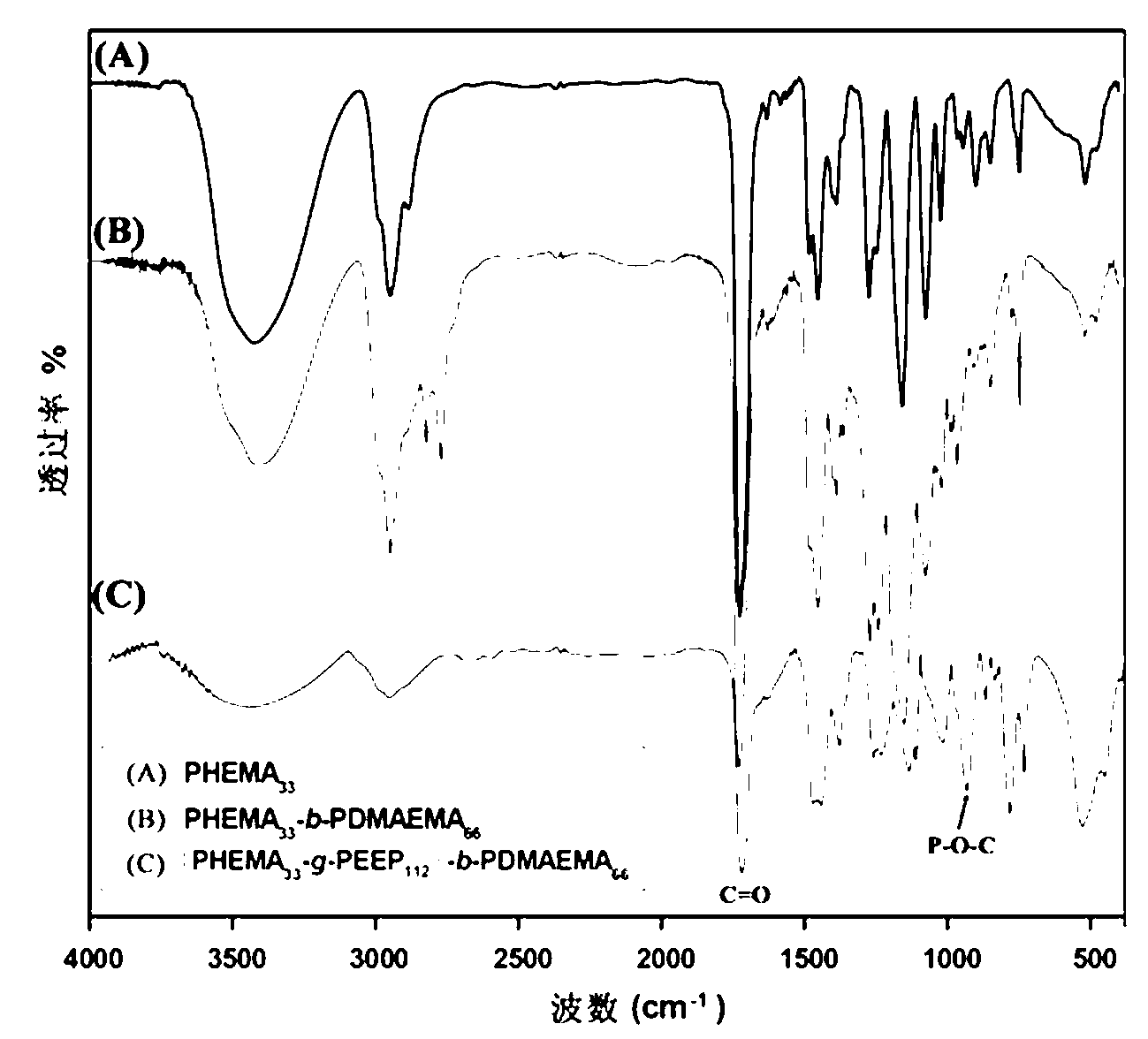
Cationic brush block copolymer and preparation method as well as application thereof
InactiveCN103059236APromote degradationGood biocompatibilityOther foreign material introduction processesPolymer science(Hydroxyethyl)methacrylate
The invention discloses a cationic brush block copolymer and a preparation method as well as an application of the block copolymer. The cationic brush block copolymer of which a side chain contains biodegradable polyphosphonate is prepared by combining an ATRP (atom transfer radical polymerization) with an ROP (ring-opening polymerization) for the first time; and the preparation method comprises the following steps of: firstly, carrying out ATRP polymerization reaction on hydroxyethyl methacrylate by a micromolecle ATRP initiator, thus obtaining macromolecule with bromine or chlorine substituent at the tail end; carrying out ATRP polymerization reaction between the macromolecule serving as a macromolecular ATRP initiator and a monomer sensitive to PH to obtain a PH response diblock copolymer; and carrying out ring-opening and graft copolymerization on a ring phosphate monomer by using hydroxyl on the side group of the diblock copolymer, thus obtaining the cationic brush block copolymer of which the side chain contains polyphosphonate. The novel cationic brush block copolymer of which the side chain contains polyphosphonate has good biocompatibility and biodegradability and low toxicity, so that the block copolymer can be used as a gene vector, and has good application prospect in a biomedicine.
Owner:SUZHOU UNIV
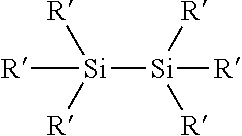
Process for the production of organohydridochlorosilanes
The invention relates to a process for the manufacture of organomonosilanes, in particular, bearing both hydrogen and chlorine substituents at the silicon atom by subjecting a silane substrate comprising one or more organomonosilanes, with the proviso that at least one of these silanes has at least one chlorine substituent at the silicon atom, to the reaction with one or more metal hydrides selected from the group of an alkali metal hydride and an alkaline earth metal hydride in the presence of one or more compounds (C) acting as a redistribution catalyst.
Owner:MOMENTIVE PERFORMANCE MATERIALS INC
Process for the production of organohydridochlorosilanes
The invention relates to a process for the manufacture of organomonosilanes, in particular, bearing both hydrogen and chlorine substituents at the silicon atom by subjecting a silane substrate comprising one or more organomonosilanes, with the proviso that at least one of these silanes has at least one chlorine substituent at the silicon atom, to the reaction with one or more metal hydrides selected from the group of an alkali metal hydride and an alkaline earth metal hydride in the presence of one or more compounds (C) acting as a redistribution catalyst.
Owner:MOMENTIVE PERFORMANCE MATERIALS INC
Functionalized copolymers of terminally functionalized perfluoro (alkyl vinyl ether) reactor wall for photochemical reactions, process for increasing fluorine content in hydrocarbons and halohydrocarb
InactiveCN101090767APreparation by hydrogen halide split-offCatalyst activation/preparationVinyl etherHalohydrocarbon
A photochemical reaction apparatus including a reactor and a light source situated so that light from the light source is directed through a portion of the reactor wall is disclosed. The apparatus is characterized by the portion of the reaction wall comprising a functionalized copolymer of a terminally functionalized perfluoro (alkyl vinyl ether). Also described is a photochemical reaction process using said reactor. The functional group of the copolymer of the apparatus and the process is selected from -SO2F, -SO2CI, -SO3H, -CO2R (where R is H or C1-C3 alkyl), -PO3H2, and salts thereof. A process for increasing the fluorine content of at least one compound selected from hydrocarbons and halohydrocarbons, comprising: (a) photochlorinating said at least one compound, and (b) reacting the halogenated hydrocarbon in (a) with HF. A process for producing an olefinic compound, comprising: (a) photochlorinating at least one compound selected from hydrocarbons and halohydrocarbons containing at least two carbon atoms and at least two hydrogen atoms to produce a halogenated hydrocarbon containing a hydrogen substituent and a chlorine substituent on adjacent carbon atoms; and (b) subjecting the halogenated hydrocarbon produced in (a) to dehydrohalogenation.
Owner:EI DU PONT DE NEMOURS & CO
Cationic brush block copolymer and preparation method as well as application thereof
InactiveCN103059236BPromote degradationGood biocompatibilityOther foreign material introduction processesSide chainPhosphate
Owner:SUZHOU UNIV
Copper chloride phthalocyanine pigment composition for non-bronze plastic coloring and preparation method
ActiveCN111763433BUniform color distributionStrong covering powerPorphines/azaporphinesSulfonateImide
Owner:浙江瑞灿科技有限公司
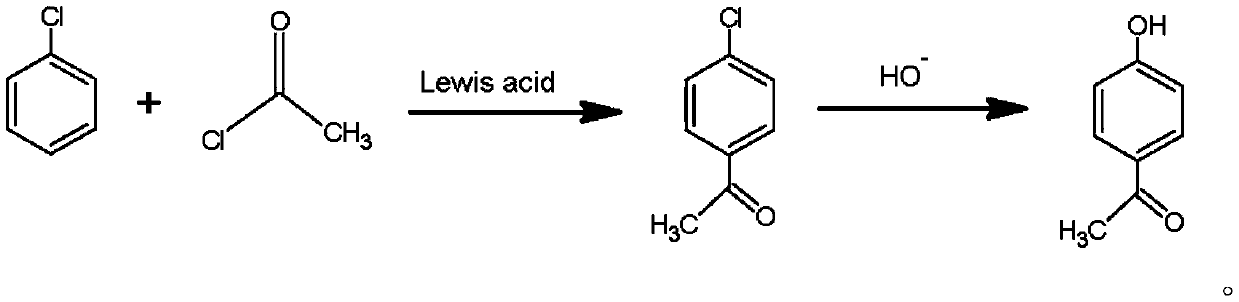
Preparation method of p-hydroxyacetophenone
PendingCN111056929AEliminate the separation and recovery processHigh yieldOrganic compound preparationCarbonyl compound preparation by condensationChlorobenzeneAcetophenone
The invention provides a preparation method of p-hydroxyacetophenone. Chlorobenzene and acetyl chloride are used as reaction substrates; a Fries rearrangement reaction; acetyl is connected to the para-position of chlorobenzene; chlorine substituents on a benzene ring are hydrolyzed to hydroxyl groups by hydrolysis reaction, and thus the target product is obtained. The preparation method provided by the invention overcomes the problem of more byproducts o-hydroxyacetophenone in a conventional technology, and has the advantages of simple process, fewer types of used raw materials, no excessive discharge of three wastes in the post-treatment process, and good production and application prospects.
Owner:老河口瑞祥化工有限公司
Process for production of 2,3-dichlorobutadiene-1,3
ActiveUS8026400B2Preparation by dehalogenationPreparation by hydrogen halide split-offChlorine substituentDichlorobutadiene
Purified chlorinated alkenes are produced by a process in which a mixture of i) a first chlorinated alkene that has at least one beta-chlorine substituent and no alpha-chlorine substituents and ii) a second chlorinated alkene that has at least one alpha-chlorine substituent is contacted with chlorine in an amount sufficient to further chlorinate the second chlorinated alkene, but which is insufficient to cause conversion of more than 20% of the first chlorinated alkene. The resultant reaction product may be easily enriched to provide a chlorinated alkene product wherein a) the weight percentage of chlorinated alkenes having at least one beta-chlorine substituent and no alpha-chlorine substituents, based on the total weight of the chlorinated alkenes present in the enriched chlorinated alkene product compared to b) the weight percentage of chlorinated alkenes having at least one beta-chlorine substituent and no alpha-chlorine substituents, based on the total weight of the chlorinated alkenes present in the mixture prior to chlorination is increased by at least 0.25 wt. %.
Owner:DENKA PERFORMANCE ELASTOMER LLC
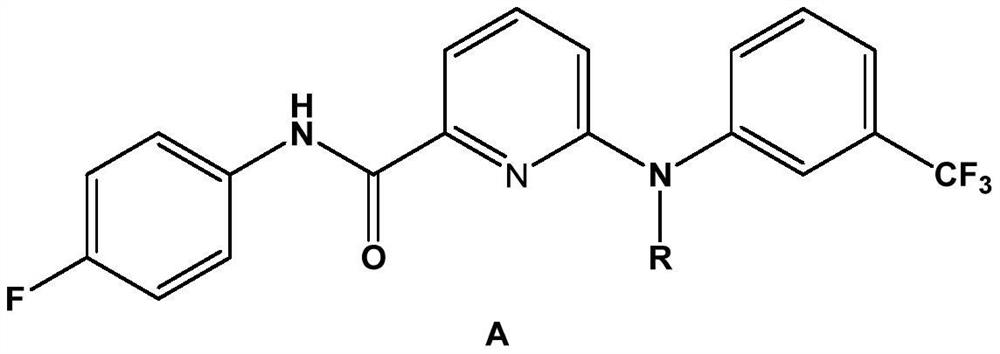
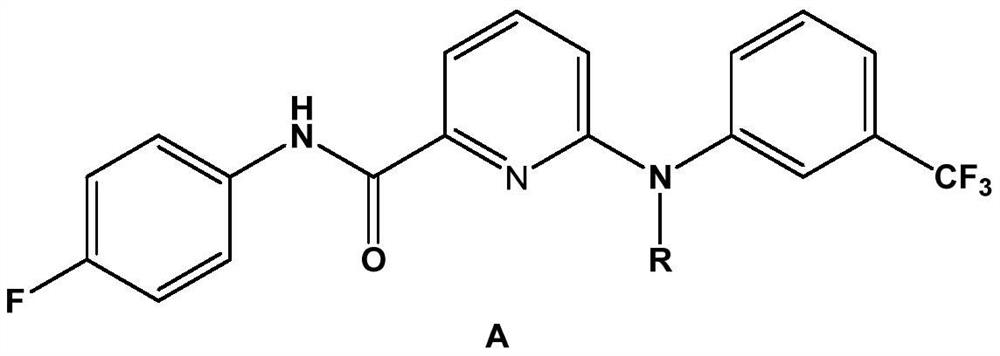
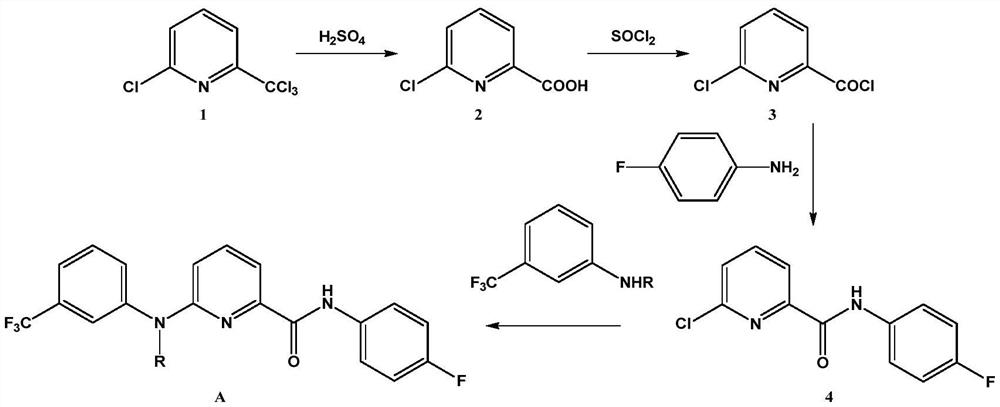
Picolinafen derivative as well as preparation method and application thereof
The invention belongs to the field of pesticide chemical industry, and relates to a picolinafen derivative as well as a preparation method and application thereof, the structural formula of the picolinafen derivative is shown in the specification, and the preparation method comprises the following steps: (1) preparing 2-chloro-6-carboxypyridine from 2-chloro-6-trichloromethylpyridine under the action of concentrated sulfuric acid; (2) preparing 2-chloro-6-acyl chloride pyridine from the 2-chloro-6-carboxypyridine under the action of phosphorus trichloride, phosphorus pentachloride or thionyl chloride; and (3) carrying out amidation reaction on the 2-chloro-6-acyl chloride pyridine and p-fluoroaniline, and then substituting a chlorine substituent group in the 2-chloro-6-acyl chloride pyridine and the p-fluoroaniline with N-substituted m-trifluoromethylaniline to obtain the picolinafen derivative. The picolinafen derivative disclosed by the invention has better herbicidal activity and can be used for preparing herbicides.
Owner:CHANGZHOU UNIV
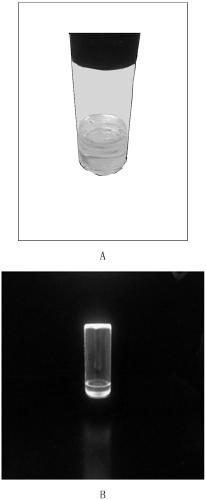
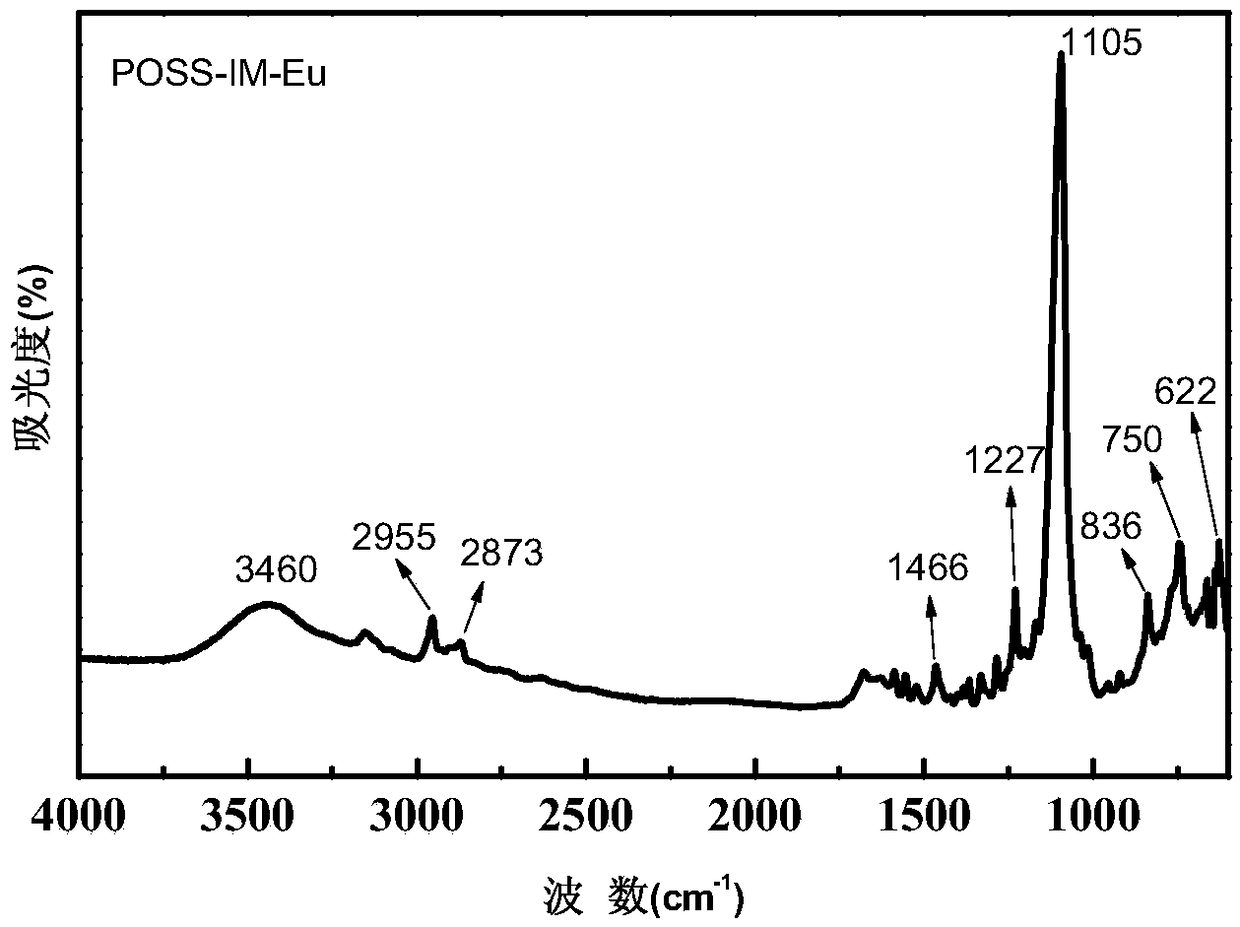
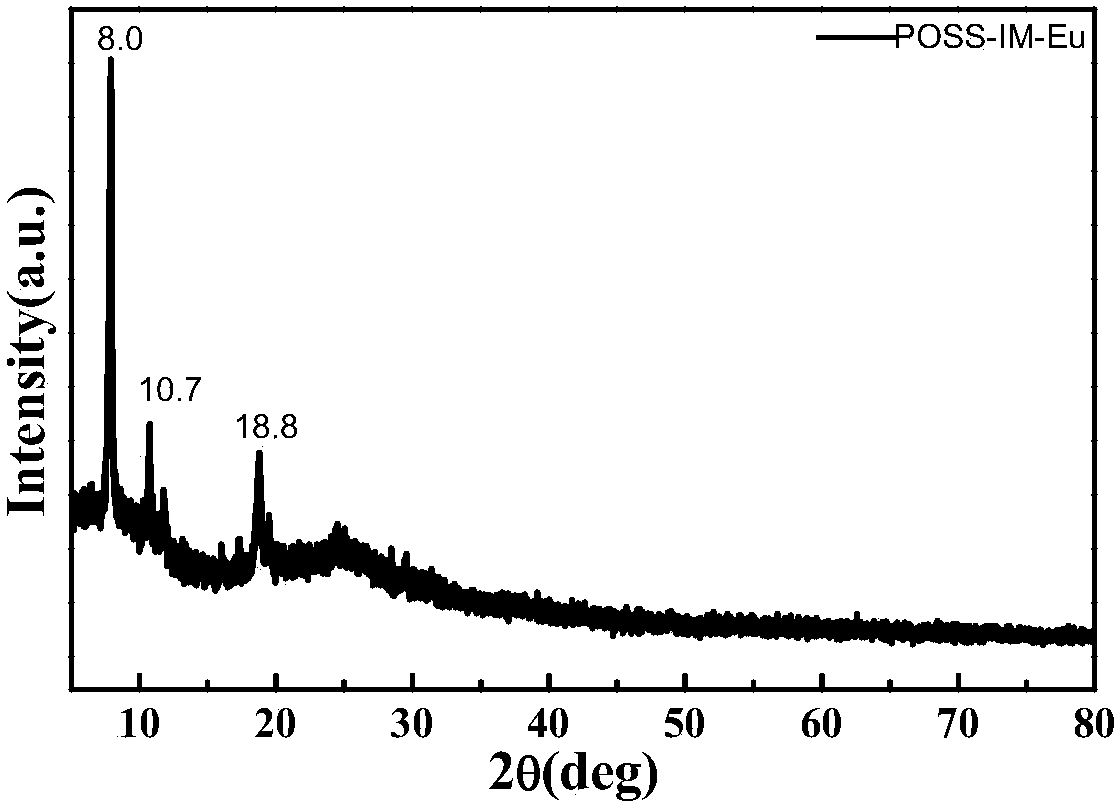
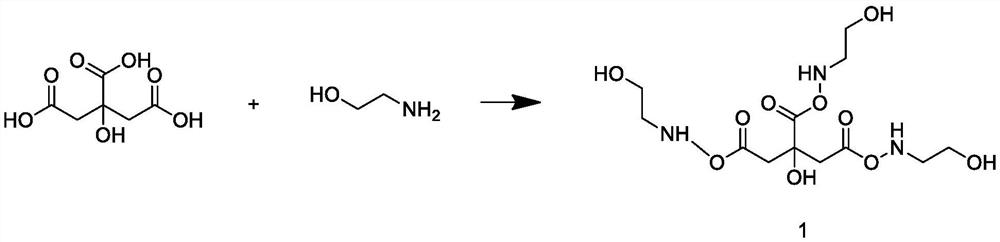
A kind of preparation method of poss base/rare earth ionic liquid fluorescent soft material
InactiveCN105969335BGood fluorescenceExcellent fluorescence performanceLuminescent compositionsFluorescenceIonic liquid
The invention provides a preparation method of a POSS (Polyhedral Oligomeric Silsesquioxane)-based / rare earth ion liquid fluorescent soft material. The preparation method comprises a step of preparing a rare earth hydrochloride ethanol solution and comprises the following steps: dissolving an organic ligand into absolute ethyl alcohol; then adding the rare earth hydrochloride ethanol solution and reacting to obtain a rare earth organic complex solution; putting amino POSS into excessive amount of thionyl chloride and reacting; purifying to obtain POSS with a chlorine substituent group; adding an ethanol solution of ion liquid and reacting under the protection of N2 to obtain a POSS functionalized ion liquid molecule bridge; in the POSS functionalized ion liquid molecule bridge, adding the rare earth organic complex solution and reacting to obtain the POSS-based / rare earth ion liquid fluorescent soft material. By grafting the polyhedral oligomeric silsesquioxane, the ion liquid and the rare earth organic complex with rare earth / ions through covalent bonds and the obtained POSS-based / rare earth ion liquid fluorescent soft material has a better fluorescent performance and the thermal stability is relatively improved.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
A paper diaper capable of effectively antibacterial and deodorizing
ActiveCN113289047BImprove adsorption capacityImprove stabilityOrganic chemistryAbsorbent padsPolymer scienceSide chain
The invention discloses a paper diaper capable of effectively antibacterial and deodorizing, which belongs to the technical field of paper diapers; it includes a surface layer, a diversion layer, toilet paper, an absorbent core layer and a bottom film, and the absorbent core layer includes the following raw materials: starch, polyacrylamide, Linking agent, initiator, fluff pulp, bamboo charcoal fiber, silver nitrate and antibacterial debonding agent; use citric acid and ethanolamine to react to produce intermediate product 1, and then react with thionyl chloride to obtain four chlorine substituents Intermediate product 2, one molecule of intermediate product 2 can undergo quaternization reaction with four molecules of N,N-dimethyloctyl tertiary amine, and more nitrogen positive ions can increase its antibacterial effect. The alkyl chains with longer chains are hydrophobic, which can reduce the combination of hydrogen bonds, and use electrostatic adsorption to attach to the surface of fluff pulp fibers, reducing the static friction coefficient between fibers, so that the product has a certain degree of softness.
Owner:礼顿卫生用品(佛山)有限公司
Paper diaper capable of effectively resisting bacteria and deodorizing
ActiveCN113289047AImprove adsorption capacityImprove stabilityOrganic chemistryAbsorbent padsPolymer scienceSide chain
The invention discloses a paper diaper capable of effectively resisting bacteria and deodorizing, and belongs to the technical field of paper diapers. The invention comprises a surface layer, a flow guide layer, toilet paper, an absorption core layer and a bottom film, and the absorption core layer is prepared from starch, polyacrylamide, a cross-linking agent, an initiator, fluff pulp, bamboo charcoal fibers, silver nitrate and an antibacterial debonding agent; citric acid and ethanolamine react to prepare an intermediate product 1, then the intermediate product 1 reacts with thionyl chloride to prepare an intermediate product 2 with four chlorine substituent groups, the single-molecule intermediate product 2 can be subjected to quaternization reaction with four-molecule N, N-dimethyl octyl tertiary amine, more nitrogen positive ions can improve the antibacterial effect of the antibacterial agent. A relatively long alkyl chain of a side chain of the antibacterial debonding agent has hydrophobicity, can reduce combination of hydrogen bonds, and is attached to the surfaces of fluff pulp fibers by utilizing an electrostatic adsorption effect to reduce a static friction coefficient between the fibers, so that the product has certain softness.
Owner:礼顿卫生用品(佛山)有限公司
Chlorine substituent modified color water-permeable asphalt and preparation method thereof
InactiveCN108083689AIncrease carbon residual rateImprove heat resistanceFree energiesChlorine substituent
The invention relates to the technical field of preparation of asphalt, in particular to chlorine substituent modified color water-permeable asphalt which has relatively good adhesive property. The chlorine substituent modified color water-permeable asphalt is prepared from the following components in parts by weight: 100 parts of mineral aggregate, 60 to 80 parts of colored stone, 50 to 80 partsof asphalt, 70 to 90 parts of petroleum resin, 3 to 10 parts of coupling agent, 1 to 3 parts of cross-linking agent, and 0.05 to 2 parts of chlorine substituent modifier. By virtue of the technical scheme provided by the invention, chlorine atoms are introduced onto a polymer molecular chain of asphalt by virtue of the chlorine substituent modifier, so that the polarity of the polymer is improved,the compatibility of the asphalt with other substances is improved, the free energy of the surface of the asphalt is changed by virtue of a chemical combination way, and the adhesive strength betweenthe asphalt and other interfaces can be improved.
Owner:南通鸿基市政工程有限公司
Process for the production of organohydridochlorosilanes
The invention relates to a process for the manufacture of organomonosilanes bearing both hydrogen and chlorine substituents at the silicon atom by subjecting a silane substrate comprising one or more silanes selected from organomonosilanes, organodisilanes and organocarbodisilanes, with the proviso that at least one of these silanes has at least one chlorine substituent at the silicon atom, to a redistribution reaction in the presence of a phosphane or amine acting as a redistribution catalyst.
Owner:MOMENTIVE PERFORMANCE MATERIALS INC
Chlorine-containing 2,4,6-trinitro-1,3-distyrylbenzene derivatives, preparation and application
InactiveCN104016868BEasy to passAvoid decompositionOrganic chemistryOrganic compound preparationChemical structureOrtho position
Owner:NANJING UNIV OF SCI & TECH
Process for production of 2,3-dichlorobutadiene-1,3
ActiveUS20090163746A1Preparation by dehalogenationPreparation by hydrogen halide split-offChlorine substituentDichlorobutadiene
Purified chlorinated alkenes are produced by a process in which a mixture of i) a first chlorinated alkene that has at least one beta-chlorine substituent and no alpha-chlorine substituents and ii) a second chlorinated alkene that has at least one alpha-chlorine substituent is contacted with chlorine in an amount sufficient to further chlorinate the second chlorinated alkene, but which is insufficient to cause conversion of more than 20% of the first chlorinated alkene. The resultant reaction product may be easily enriched to provide a chlorinated alkene product wherein a) the weight percentage of chlorinated alkenes having at least one beta-chlorine substituent and no alpha-chlorine substituents, based on the total weight of the chlorinated alkenes present in the enriched chlorinated alkene product compared to b) the weight percentage of chlorinated alkenes having at least one beta-chlorine substituent and no alpha-chlorine substituents, based on the total weight of the chlorinated alkenes present in the mixture prior to chlorination is increased by at least 0.25 wt. %.
Owner:DENKA PERFORMANCE ELASTOMER LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com