Cationic triblock copolymer based on biodegradable polyphosphate ester and application thereof
A polyphosphate, cationic technology, applied in other methods of inserting foreign genetic materials, recombinant DNA technology, etc., can solve the problems of strong crystallinity, unfavorable degradation, and large hydrophobicity of polyester, and achieve good biocompatibility and the effect of biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
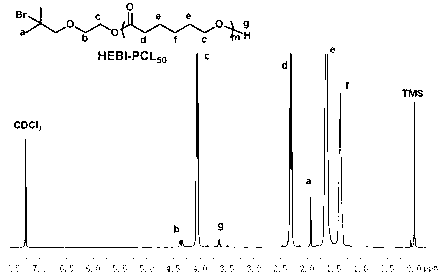
[0062] Embodiment one: macromolecular initiator HO-PCL 50 The specific synthesis method of -X
[0063] Dry the branched round-bottomed flask with a stirrer in an oven at 120°C for at least 24 hours, plug it with a glass stopper, connect it to an oil pump through a latex tube and pump it to room temperature, then introduce high-purity argon gas, and then evacuate it, repeat this process three times.
[0064] Inject 2-hydroxyethyl bromoisobutyrate (HEBI) (0.12 g, 0.57 mmol), caprolactone (ε-CL) (3.89 g, 34.12 mmol) and stannous octoate sequentially from the branch tube with a dry syringe (Sn(Oct) 2 ) (7.8mg) (-CL is calculated according to the theoretical degree of polymerization; ε-CL:Sn(Oct) 2 =1:0.002, mass ratio), 10mL of anhydrous toluene as solvent. After the reaction flask was filled with argon, the reaction was stirred in an oil bath at 90° C. for 12 h.
[0065] After the reaction was completed, most of the toluene solvent was removed by rotary evaporation, and unre...
Embodiment 2
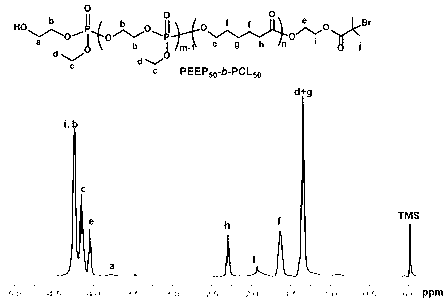
[0066] Example 2: Diblock copolymer PEEP containing polyphosphate 50 -b-PCL 50 synthesis method
[0067] After treating the branch tube round bottom flask according to the above method, fill it with argon gas, weigh it quickly and add a certain amount of HEBI-PCL 50 (3g, 0.53mmol) and a small amount of stannous octoate (6mg), inject 10mL tetrahydrofuran with a dry syringe to completely dissolve HEBI-PCL, and then inject a certain amount of monomer 2-ethoxy-2-oxo-1, 3,2-Dioxaphospholane (EEP) (5.52g, 34.45mmol) (EEP:Sn(Oct) 2 =1:0.002, mass ratio), the round bottom flask was filled with argon and moved to a 40°C oil bath to stir for 3h.
[0068] After the reaction, a large amount of tetrahydrofuran was removed by rotary evaporation, and the unreacted monomer and homopolymer were removed by precipitation with 150 mL ice anhydrous ether / ice methanol (volume ratio 10:1). The product obtained after suction filtration on a Buchner funnel was placed in a vacuum oven and dried to ...
Embodiment 3
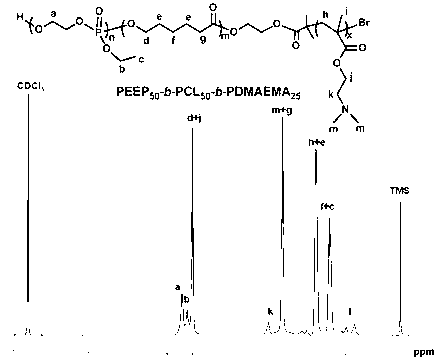
[0069] Embodiment three: cationic triblock copolymer PEEP 50 -b-PCL 50 -b-PDMAEMA 25 synthesis method
[0070]After the branch tube round bottom flask was treated according to the above method, it was filled with argon, and the above two-block polymer PEEP was quickly weighed and added. 50 -b-PCL 50 (4g, 0.30mmol), cuprous bromide (0.042g, 0.30mmol) and bipyridine (0.187g, 0.6mol) (the molar ratio of the three is 1:1:2), and the exhaust gas is repeated three times to ensure that the system is free of oxygen Add 10 mL of solvent N,N-dimethylformamide (DMF) with a dry syringe, and stir until the mixture is completely dissolved and the solution turns dark brown.
[0071] Then the round bottom flask was moved to an oil bath at 50°C, and 2-(dimethylamino)ethyl methacrylate DMAEMA (1.884g, 12mmol) was added with a dry syringe (calculated according to the theoretical degree of polymerization). The halogen at the end of the PCL segment in the diblock acts as an initiator to initi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com