Cationic brush block copolymer and preparation method as well as application thereof
A block copolymer, cationic technology, applied in cationic brush-shaped block copolymer, its preparation field, can solve the problem of increasing the toxicity of the carrier, achieve good application value, reduce toxicity, improve the effect of distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
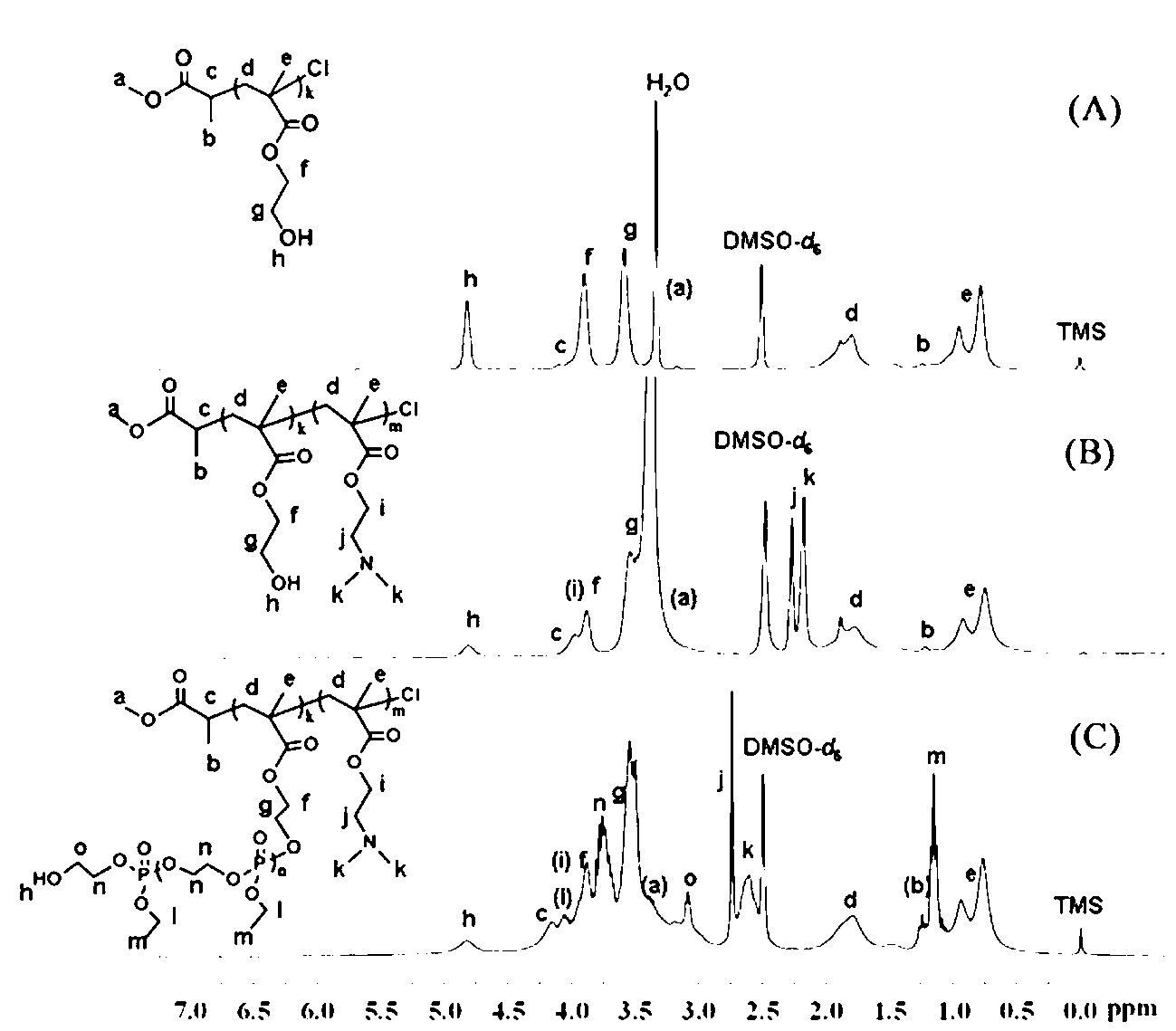
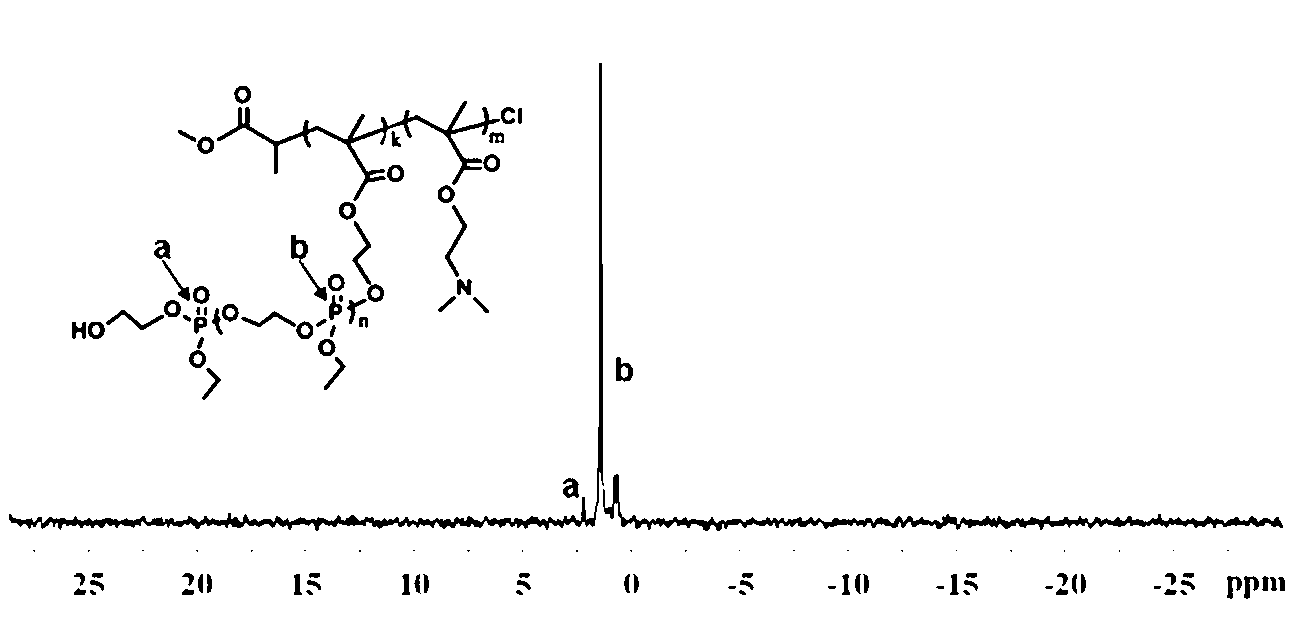
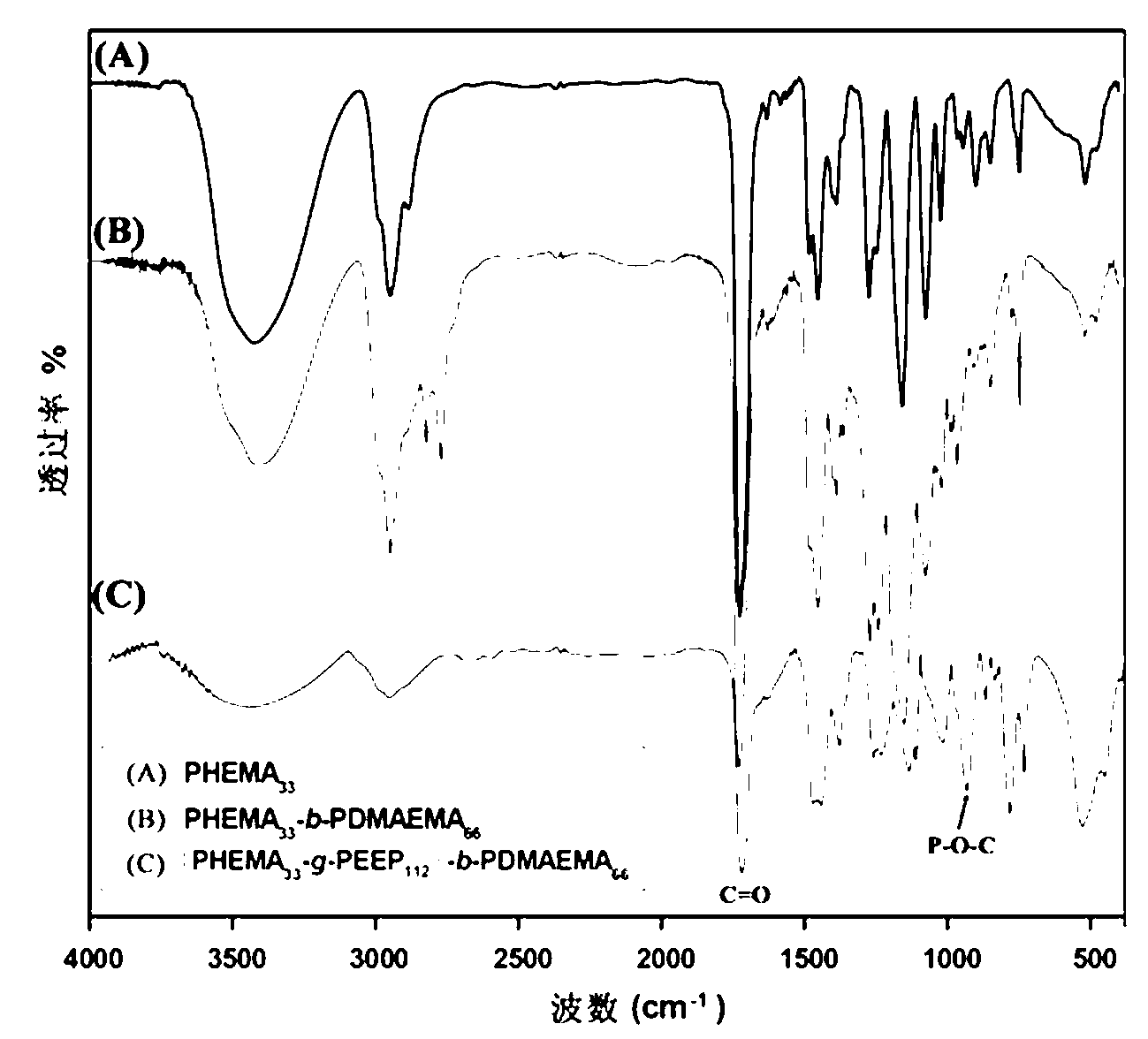
[0043] Embodiment one: ATRP macromolecular initiator polyhydroxyethyl methacrylate PHEMA 33 Synthesis of -Cl
[0044] Put the branch tube round bottom flask in an oven at 120 ℃ to dry, then plug it with a ground glass stopper, pump it to cool until it reaches room temperature, fill it with argon after three times of filling and degassing. The monomer hydroxyethyl methacrylate (HEMA) (4.1 g, 31.50 mmol) was dissolved in methanol (10 mL) and added to the vial, and the small molecule initiator methyl α-bromopropionate (119 mu L, 1.05 mmol), ligand 2,2′-bipyridine (328.0 mg, 2.1 mmol), cuprous chloride (104.0 mg, 1.05 mmol) with a purity of 99.99% were added to the above mixed system, and argon was introduced Protect. Raise the temperature to 30°C, and maintain this temperature, stir with magnetic force, and react for 10-12 hours.
[0045] Methanol was added to dilute the reaction product, and it was contacted with air while stirring to terminate the reaction. After the solut...
Embodiment 2
[0047] Example 2: Diblock copolymer PHEMA with side groups containing hydroxyl groups 33 - b -PDMAEMA 66 Synthesis
[0048] Put the branch tube round bottom flask in an oven at 120 ℃ to dry, then plug it with a ground glass stopper, pump it to cool down until it reaches room temperature, fill and discharge the argon gas three times, and then continue to fill the glass bottle with argon gas. The macroinitiator PHEMA that embodiment one prepares 33 -Cl (2.2 g, 0.5 mmol) dissolved in methanol (15 mL) was added to the vial, the ligand 2,2′-bipyridine (156.2 mg, 1.0 mmol), the monomer methacrylic acid-2-(dimethyl Amino)ethyl ester (DMAEMA) (4.6 g, 30 mmol) and cuprous chloride (49.5 mg, 0.5 mmol) with a purity of 99.99% were added to the above mixed system, and argon protection was introduced. Raise the temperature to 35°C, and maintain this temperature, stir with magnetic force, and react for 24-28 hours.
[0049] Methanol was added to dilute the reaction product, and it was ...
Embodiment 3
[0051] Example 3: Diblock copolymer PHEMA with side groups containing hydroxyl groups 33 - b -PDMAEMA 50 Synthesis
[0052] Put the branch tube round bottom flask in an oven at 120 ℃ to dry, then plug it with a ground glass stopper, pump it to cool down until it reaches room temperature, fill and discharge the argon gas three times, and then continue to fill the glass bottle with argon gas. The macroinitiator PHEMA that embodiment one prepares 33-Cl (2.2 g, 0.5 mmol) dissolved in methanol (15 mL) was added to the vial, the ligand 2,2′-bipyridine (156.2 mg, 1.0 mmol), the monomer methacrylic acid-2-(dimethyl Amino)ethyl ester (DMAEMA) (3.8 g, 25 mmol) and cuprous chloride (CuCl, 49.5 mg, 0.5 mmol) with a purity of 99.99% were added to the above mixed system, and argon gas protection was introduced. Raise the temperature to 35°C, and maintain this temperature, stir with magnetic force, and react for 24-28 hours.
[0053] Methanol was added to dilute the reaction product, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com