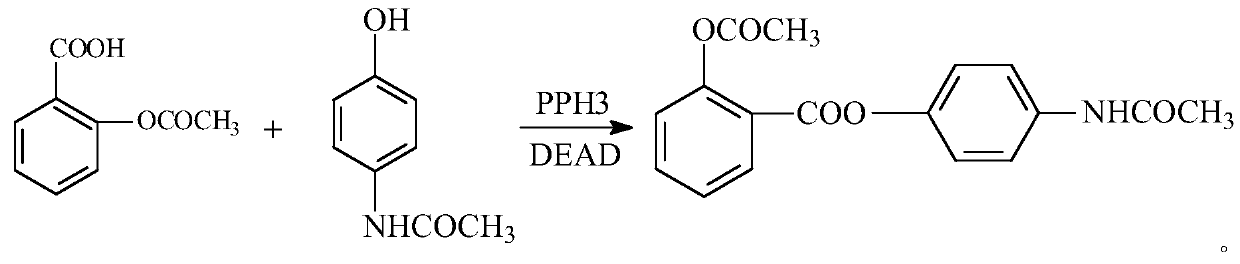
Preparation method of benorilate
A technology for the reaction of Benodate and Mitsunobu is applied in the field of preparation of Benodate, which can solve the problems of high price, low yield, large solvent consumption, etc., and achieve the effects of reducing synthesis cost, mild synthesis conditions and easy control of reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Under ice-water bath conditions, in a 50mL flask, first add 0.3023g of acetaminophen and 0.6295g of triphenylphosphine, then add 20mL of dichloromethane, then add 0.552g of di-tert-butyl azodicarboxylate, and finally in 20min After adding 0.4324g of acetylsalicylic acid step by step, after keeping it in an ice-water bath for 20 minutes, return to normal temperature for reaction. The reaction time is 8.5 hours, and the yield is 63.0%. Crystallization and separation are carried out with 95% ethanol, and after three times of recrystallization, the obtained White beinolate crystals, the total yield is 52.6% based on acetaminophen. The product quality conforms to the 2005 edition of "Chinese Pharmacopoeia".
Embodiment 2
[0024] Under ice-water bath conditions, first add 0.3023g of acetaminophen and 0.6295g of triphenylphosphine into a 50ml flask, then add 20mL of tetrahydrofuran, then add 0.552g of di-tert-butyl azodicarboxylate, and finally add it step by step within 20min. After adding 0.4324g of acetylsalicylic acid, keep it in an ice-water bath for 20 minutes, then return to normal temperature for reaction, the reaction time is 8.5 hours, and the yield is 63.0%; use 95% ethanol to carry out crystallization and separation, and after three times of recrystallization, white Benoy is obtained Ester crystals, with a total yield of 46.3% based on acetaminophen. The product quality conforms to the 2005 edition of "Chinese Pharmacopoeia".
Embodiment 3
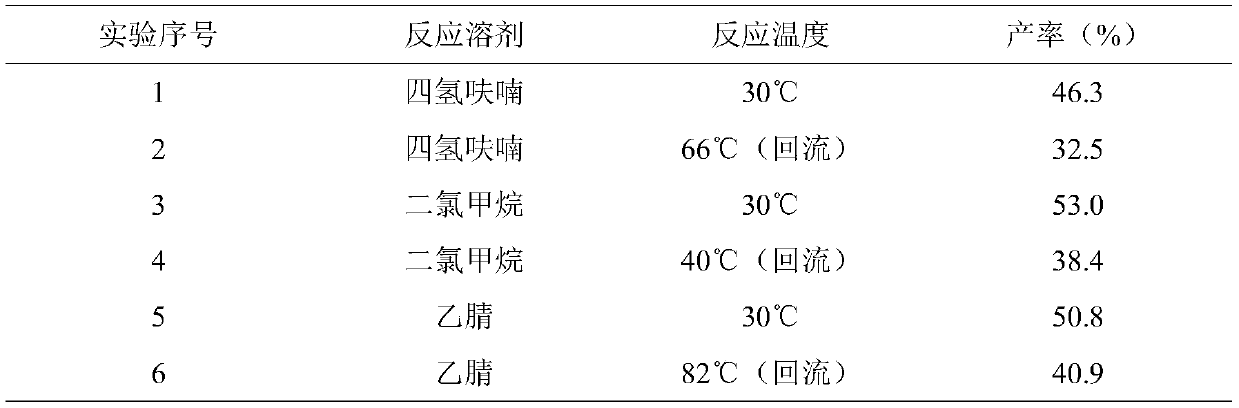
[0026] According to the measurement relationship, six parts of a certain amount of acetaminophen, acetylsalicylic acid, diethyl azodicarboxylate (DEAD) and triphenylphosphine were weighed.
[0027] Under the condition of ice-water bath, add acetaminophen and triphenylphosphine in turn to a 50mL round-bottomed flask; add 20mL of reaction solvent according to Table 1, and mix well. After the solution becomes clear, slowly add DEAD dropwise. Add acetylsalicylic acid in batches, keep in an ice-water bath for 20 minutes, heat to the reaction temperature according to Table 1, react for 8.5 hours, and calculate the yield.
[0028] As can be seen from Table 1, changing the reaction solvent and the reaction temperature affects the yield of the product beinolate; the yields of the three solvents are all higher than the yields of the high-temperature reaction at low temperatures, indicating that this reaction is optimal at room temperature. The boiling point of dichloromethane is too low...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com