Multi-channel mass spectrometry derivatization reagent for detecting sphingosine glucoside and sphingosine galactoside and its preparation method and application
A technology of sphingosine galactoside and sphingosine, which is applied in measurement devices, organic chemistry, instruments, etc., can solve the problem of inability to achieve high-throughput detection of GlcS and GalS, high price of isotopic internal standards, and high sensitivity and low sensitivity. problems such as poor accuracy, to achieve the effect of stable derivative products, high accuracy and fast response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
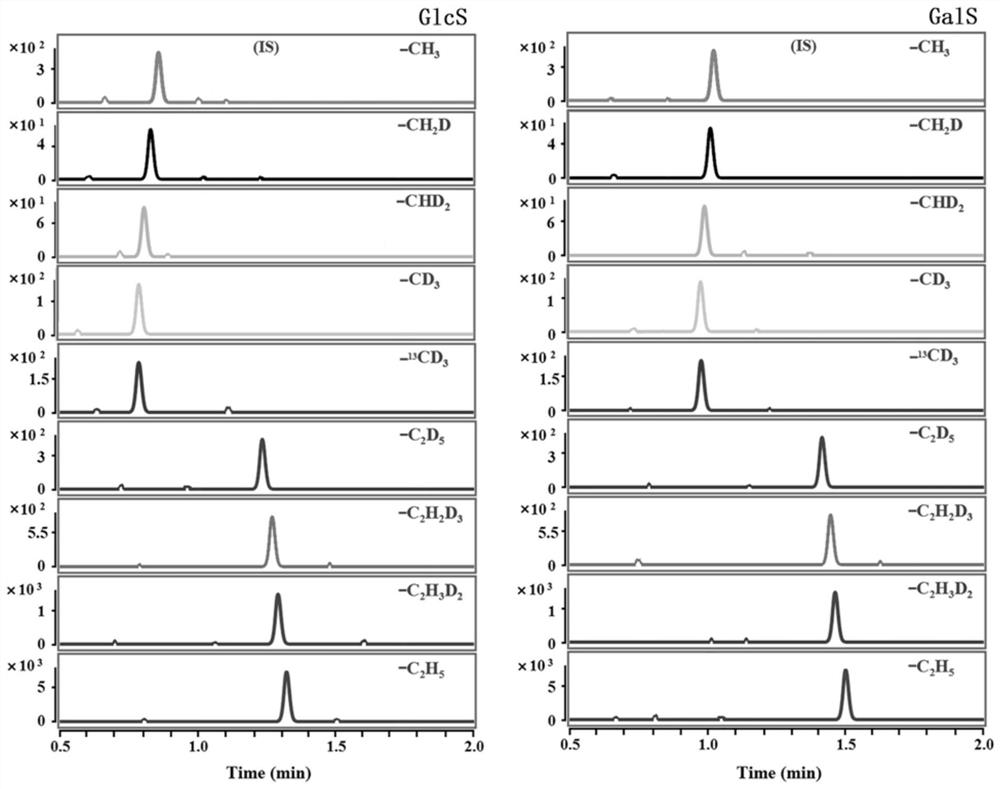
[0044] Chromatographic separation and qualitative and quantitative analysis of GlcS and GalS spiked samples in delipidated plasma:
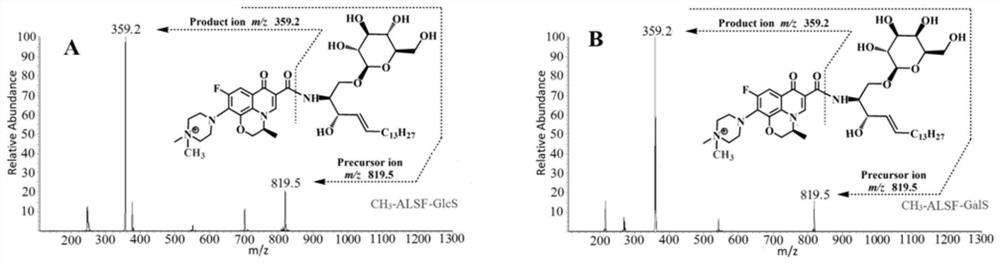
[0045] GlcS and GalS were prepared in acetonitrile to obtain 1×10 -6 Mo / L standard stock solution, and then prepared with fat-free plasma to obtain concentrations of 0.02 / 0.02, 0.2 / 0.2, 2.0 / 2.0, 20.0 / 20.0, 50.0 / 50.0, 100.0 / 100.0, 400.0 / 400.0, 800.0 / 800.0 The concentration range of the mixed standard solution of GlcS and GalS in nmol / L covers the concentration range of GlcS / GalS in the plasma of patients with Gaucher disease or Krabbe disease. CH 3 / CH 2 D / CHD 2 / 13 cd 3 / CD 3 / C 2 h 5 / C 2 h 3 D. 2 / C 2 h 2 D. 3 / C 2 D. 5 -ALSF was dissolved in acetonitrile to give 2.5×10 -8mol / L derivatization reagent acetonitrile solution; take 50 μL mixed standard solution from 8 parts of delipidated plasma mixed standard solution and a single part of acetonitrile mixed standard solution in a 1.5 mL centrifuge tube, add 300 μL H 3 BO 3 -Na ...
Embodiment 2
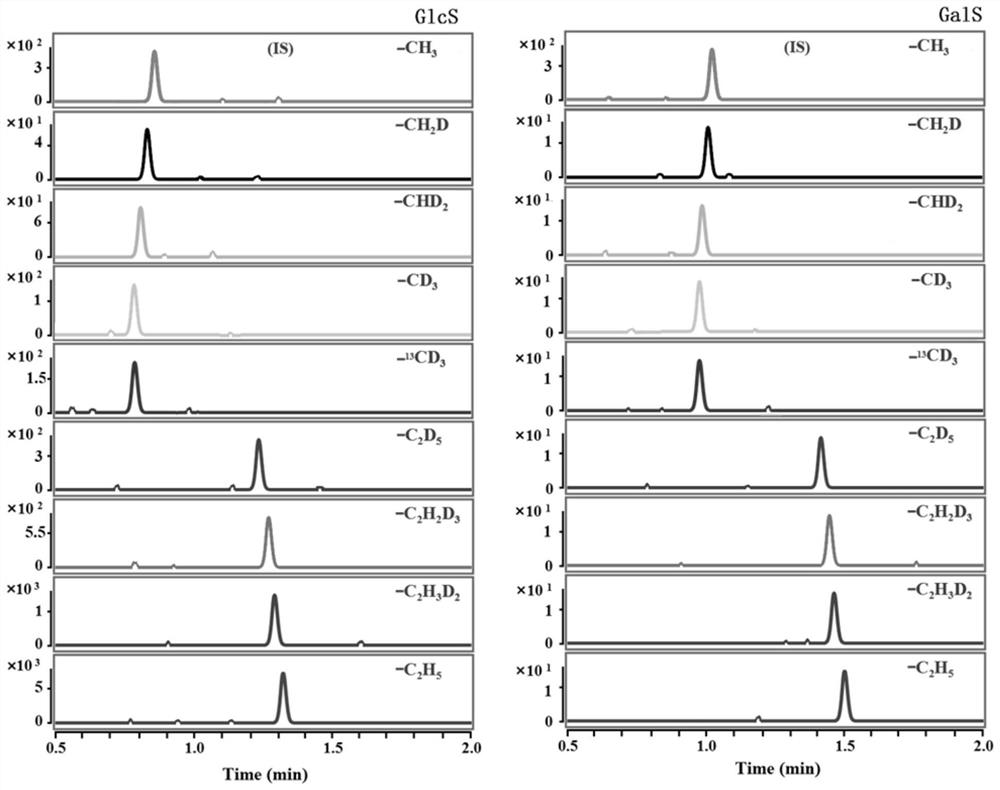
[0050] The detection and analysis of GlcS and GalS in the serum of a simulated Gaucher patient includes the following steps:
[0051] Take 8 healthy human serum samples from a local hospital, mix them evenly in a centrifuge tube, and centrifuge quickly at 4°C, take 500 μL of serum samples and add 500 μL methanol to a 1.5 mL centrifuge tube, vortex for 1.5 minutes and centrifuge to remove For protein precipitation, take 40 μL and place it in a 1.5 mL centrifuge tube, prepare 8 copies, and then add 10 μL of GlcS diluted with acetonitrile at a concentration of 25.0, 50.0, 100.0, 300.0, 500.0, 1000.0, 4000.0 nmol / L to each of the seven copies The concentration range of the standard solution after adding the standard covers the GlcS content range in the serum of patients with Gaucher disease. Add 10 μL of acetonitrile solution to the remaining portion as a control. In addition, take 50 μL of the mixed standard acetonitrile solution (from a concentration of 1×10 -8 mol / L GalS, the...
Embodiment 3
[0053] The detection of GlcS and GalS in the plasma of a simulated Gaucher patient includes the following steps:
[0054] Take 8 samples of healthy human plasma from a local hospital and mix them evenly in test tubes containing ethylenediaminetetraacetic acid (EDTA), and centrifuge them quickly at 4°C. Take 2 mL of plasma samples and add 8 mL of methanol into a 15.0 mL centrifuge tube, vortex Spin and shake for 2 minutes and centrifuge to remove protein precipitates, take 40 μL and put it in a 1.5mL centrifuge tube, prepare 8 copies, and then add 10 μL to each of the seven copies with concentrations of 25.0, 50.0, 100.0, 300.0, 500.0, 1000.0, 4000.0 GlcS standard solution diluted in nmol / L acetonitrile, the concentration range of the added solution covered the GlcS content range in the plasma of patients with Gaucher disease, and the remaining part was added with 10 μL acetonitrile solution as a control. In addition, take 50 μL of the mixed standard acetonitrile solution (from...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com