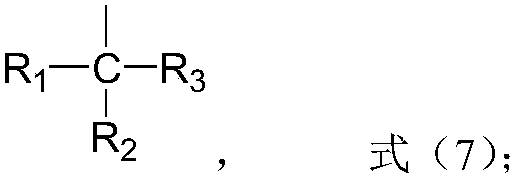
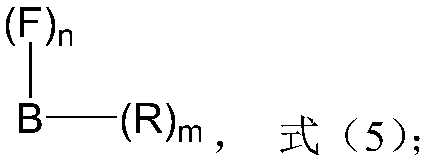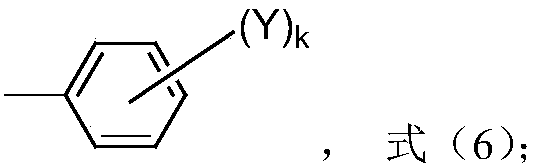Preparation method of high-activity polyether polyol
A high-activity polyether and polyol technology, which is applied in the field of preparation of high-activity polyether polyols, can solve the problems of many side reactions and low activity, and achieve the effect of simple method, high polymerization activity and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 400 g of difunctional polyether polyol GEP-204 with a molecular weight of 400 and 0.06 g of a bimetallic catalyst in a 3L autoclave equipped with a pressure and temperature gauge, a stirring device and a raw material feed port. After nitrogen replacement, the temperature was raised to 130° C., and 800 g of propylene oxide was continuously added over 1 hour. After the feeding of propylene oxide was completed, the mixture was aged for 1 hour, and the low-boiling fraction in the system was extracted with a vacuum pump, then the temperature was lowered to 60° C., and 0.06 g of tris(pentafluorophenyl)boron was added. After 1 hour, 950 g of propylene oxide was added continuously, aged for 1 hour, the low boiling point fraction in the system was extracted with a vacuum pump, 100 g of water was added, stirred for 5 hours, water was removed, and 1990 g of polymer was obtained from discharge. The polymer has a hydroxyl value of 56.3 mgKOH / g, a molecular weight distribution of...
Embodiment 2
[0047] Add 400 g of difunctional polyether polyol GEP-204 with a molecular weight of 400 and 0.06 g of a bimetallic catalyst in a 3L autoclave equipped with a pressure and temperature gauge, a stirring device and a raw material feed port. After nitrogen replacement, the temperature was raised to 130° C., and 1200 g of propylene oxide was continuously added over 1 hour. After the feeding of propylene oxide was completed, the mixture was aged for 1 hour, and the low-boiling fraction in the system was extracted with a vacuum pump, then the temperature was lowered to 60° C., and 0.06 g of tris(pentafluorophenyl)boron was added. After 1 hour, 480 g of propylene oxide was added continuously, and matured for 1 hour. The low boiling point fraction in the system was extracted with a vacuum pump, 100 g of water was added, stirred for 5 hours, water was removed, and 1990 g of polymer was discharged. The polymer has a hydroxyl value of 56.3 mgKOH / g, a molecular weight distribution of 1.07...
Embodiment 3
[0049] Add 200 g of difunctional polyether polyol GEP-204 with a molecular weight of 400 and 0.06 g of a bimetallic catalyst in a 3 L autoclave equipped with a pressure and temperature gauge, a stirring device and a raw material feed port. After nitrogen replacement, the temperature was raised to 130° C., and 1400 g of propylene oxide was continuously added over 1 hour. After the feeding of propylene oxide was completed, the mixture was aged for 1 hour, and the low-boiling fraction in the system was extracted with a vacuum pump, then the temperature was lowered to 60° C., and 0.06 g of tris(pentafluorophenyl)boron was added. After 1 hour, 500 g of propylene oxide was added continuously, aged for 1 hour, the low boiling point fraction in the system was extracted with a vacuum pump, 100 g of water was added, stirred for 5 hours, water was removed, and 1993 g of polymer was obtained from discharge. The hydroxyl value of the polymer is 28.4 mgKOH / g, the molecular weight distributi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com