Magnetic nanocomposite for specifically capturing and effectively releasing circulating tumor cells and preparation method thereof
A tumor cell and magnetic nanotechnology, applied in cell dissociation methods, tumor/cancer cells, biochemical equipment and methods, etc., can solve problems such as invasiveness, interference with cell microenvironment, damage to cell integrity structure, etc., to achieve convenient The effect of detection analysis, fast capture, and narrow particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
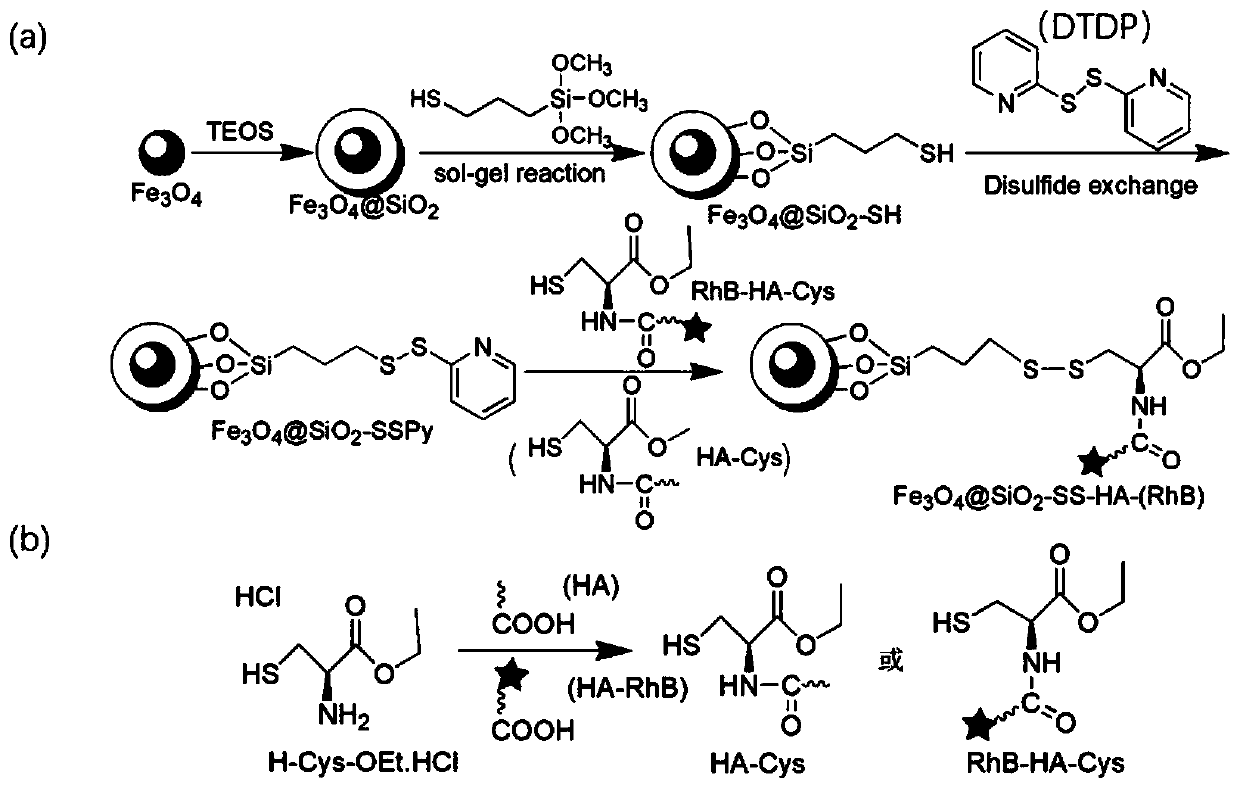
[0043] Embodiment 1 disulfide dipyridyl triiron tetroxide (Fe 3 o 4 @SiO 2 -SSPy) preparation
[0044] Take 2ml Fe 3 o 4 Nanoparticle (30mg / ml) solution, after magnetic separation, add 60ml absolute ethanol and 15ml ultrapure water, stir magnetically to make it evenly mixed, then add 1ml concentrated ammonia water, and then add 20ul tetraethyl orthosilicate every half hour Ester (TEOS), add 3 times in total, react at 40°C for 12h, then add 200ul concentrated ammonia water and 200ulmercaptopropyltrimethoxysilane (MPTMS), react at 60°C for 6h, magnetically separate the resulting product and use anhydrous Wash with ethanol for 3-4 times, and finally disperse in absolute ethanol to obtain Fe 3 o 4 @SiO 2 - SH dispersion (10 mg / ml).
[0045] Take 1ml Fe 3 o 4 @SiO 2 -SH solution was magnetically separated and dispersed in 10ml methanol, adjusted its pH to 4-5 with acetic acid, then added 2ml, 5mg / ml 2,2'-dithiobispyridylmethanol solution, stirred at room temperature for ...
Embodiment 2
[0046] Example 2 Preparation of Cysteine (Cys) Hyaluronic Acid (HA-Cys).
[0047] Dissolve hyaluronic acid HA (Mw=31000D, 310mg, 0.8mmol-COOH) in 20ml ultrapure water, then add 1-(3-methylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC (766.8mg, 4mmol) stirred for 0.5h, then added N-hydroxysuccinimide NHS (460.36mg, 4mmol), stirred for 1.5h, then added cysteine ethyl ester hydrochloride (29.70mg, 0.16mmol) After 24 hours of reaction at room temperature, the reaction solution was transferred to a dialysis bag (1000D), dialyzed with ultrapure water for 3 days, and freeze-dried to obtain the HA-Cys complex.
[0048]Dissolve RhB (95.8mg, 0.2mmol) in 10ml ultrapure water, add EDC (38.34mg, 0.2mmol), stir for half an hour, add HA (155mg, 0.4mmol) aqueous solution, then add DMAP (25mg, 0.2mmol ), react overnight at room temperature and dialyze the resulting reaction solution to remove unreacted reactants, dialyze with ultrapure water for 3 days, freeze-dry and store in the da...
Embodiment 3
[0049] Embodiment 3 magnetic (fluorescent) nanocomposite Fe 3 o 4 @SiO 2 - Preparation of SS-HA-(RhB).
[0050] Take the above-prepared HA-Cys (RhB-HA-Cys) and dissolve it in 20ml of ultrapure water. After it is completely dissolved, add 3ml (10mg / ml) of Fe under magnetic stirring 3 o 4 @SiO 2 -SSPy solution, react at room temperature for 48h, magnetically separate and wash the product several times with ultrapure water, freeze-dry to obtain Fe 3 o 4 @SiO 2 -SS-HA-(RhB) nanoparticles.
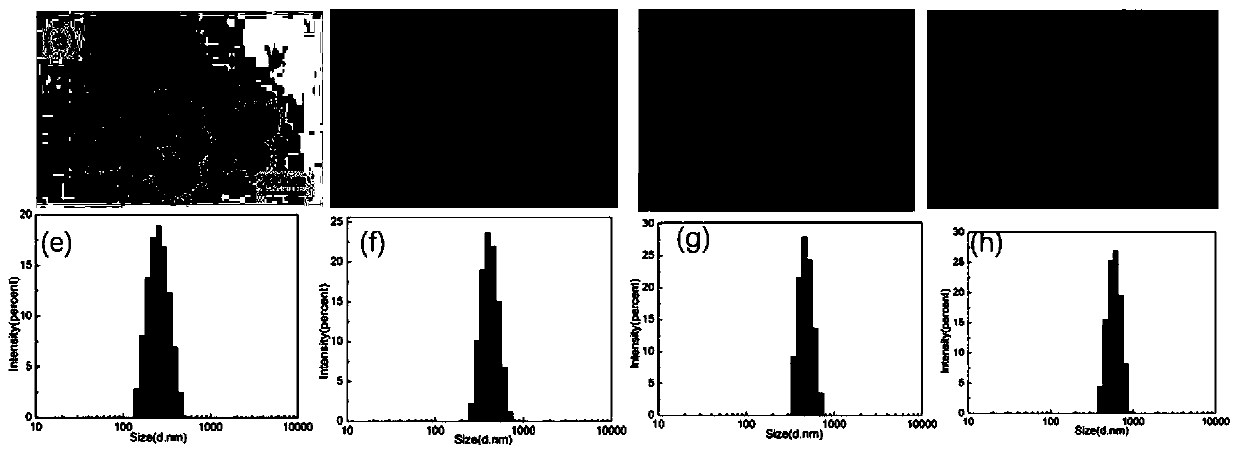
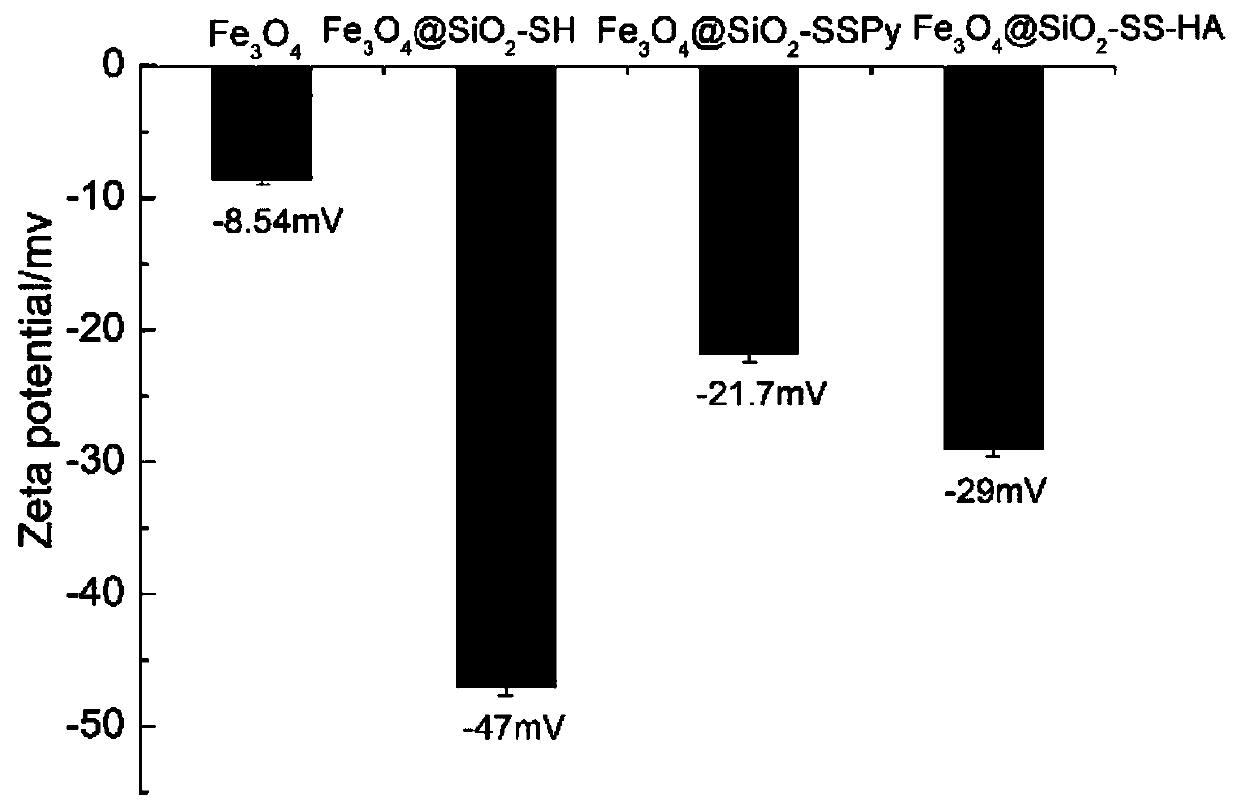
[0051] TEM images show that Fe 3 o 4 , Fe 3 o 4 @SiO 2 -SH,Fe 3 o 4 @SiO 2 -SSPy,Fe 3 o 4 @SiO 2 -SS-HA nanoparticles are spherical and uniform in size, and MPTMS(b), SSPy(c), HA(d) have been successfully modified to Fe 3 o 4 The surface of magnetic nanoparticles ( figure 2 a-d), the resulting Fe 3 o 4 @SiO 2 -SS-HA particle size distribution between 100-300nm. DLS results showed that Fe 3 o 4 , Fe 3 o 4 @SiO 2 -SH, Fe 3 o 4 @SiO 2 -SSPy, Fe 3 o 4 @SiO 2 -SS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com