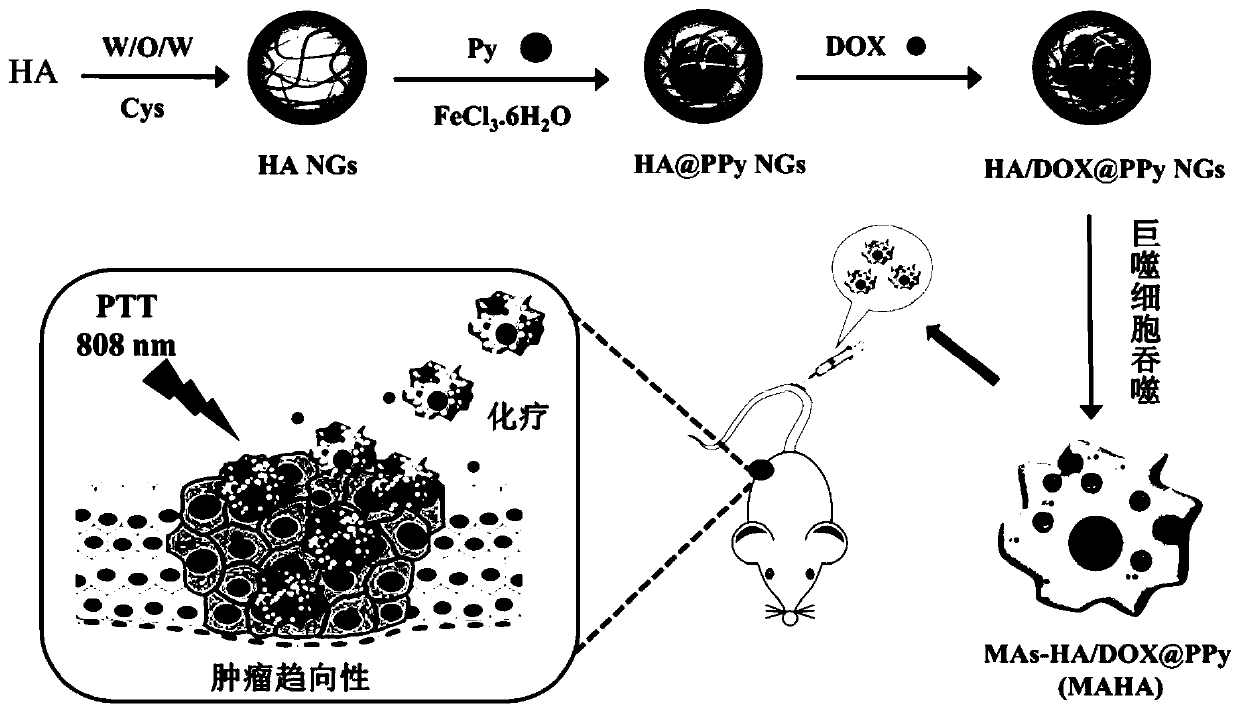
Macrophage-mediated drug-loaded hyaluronic acid nanohydrogel and preparation thereof
A nano-hydrogel and hyaluronic acid technology is applied in the field of drug-loaded nanomaterials and their preparation to achieve good tumor treatment effect, increase accumulation, and reduce endocytosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Dissolve hyaluronic acid sodium salts (20mg) with different molecular weights (48kDa, 320kDa, 950kDa, Bloomage) in 2mL of ultrapure water, add EDC (HA:EDC=1:0.5, molar ratio), magnetic The reaction was stirred for 3 h to activate the carboxyl groups on HA. The surfactant AOT (134 mg) was pre-dissolved in 4 mL of dichloromethane DCM, and then the activated HA / EDC solution was added dropwise to the AOT / DCM solution with stirring, and stirred for 10 min to form a milky white W / O emulsion , and then added the solution dropwise into a pre-dissolved PVA aqueous solution (2wt%, 30mL), and continued to stir for 15min to form a milky white W / O / W double emulsion. Finally, the cross-linking agent Cys (22.4mg, 1mL) was added to react for 1h, and then ultrasonicated in an ice bath for 10min with a sonicator, and then the bottle was opened and stirred overnight in a fume hood to completely evaporate the organic solvent DCM. The next day, the HA NGs aqueous solution was dialyzed for ...
Embodiment 2
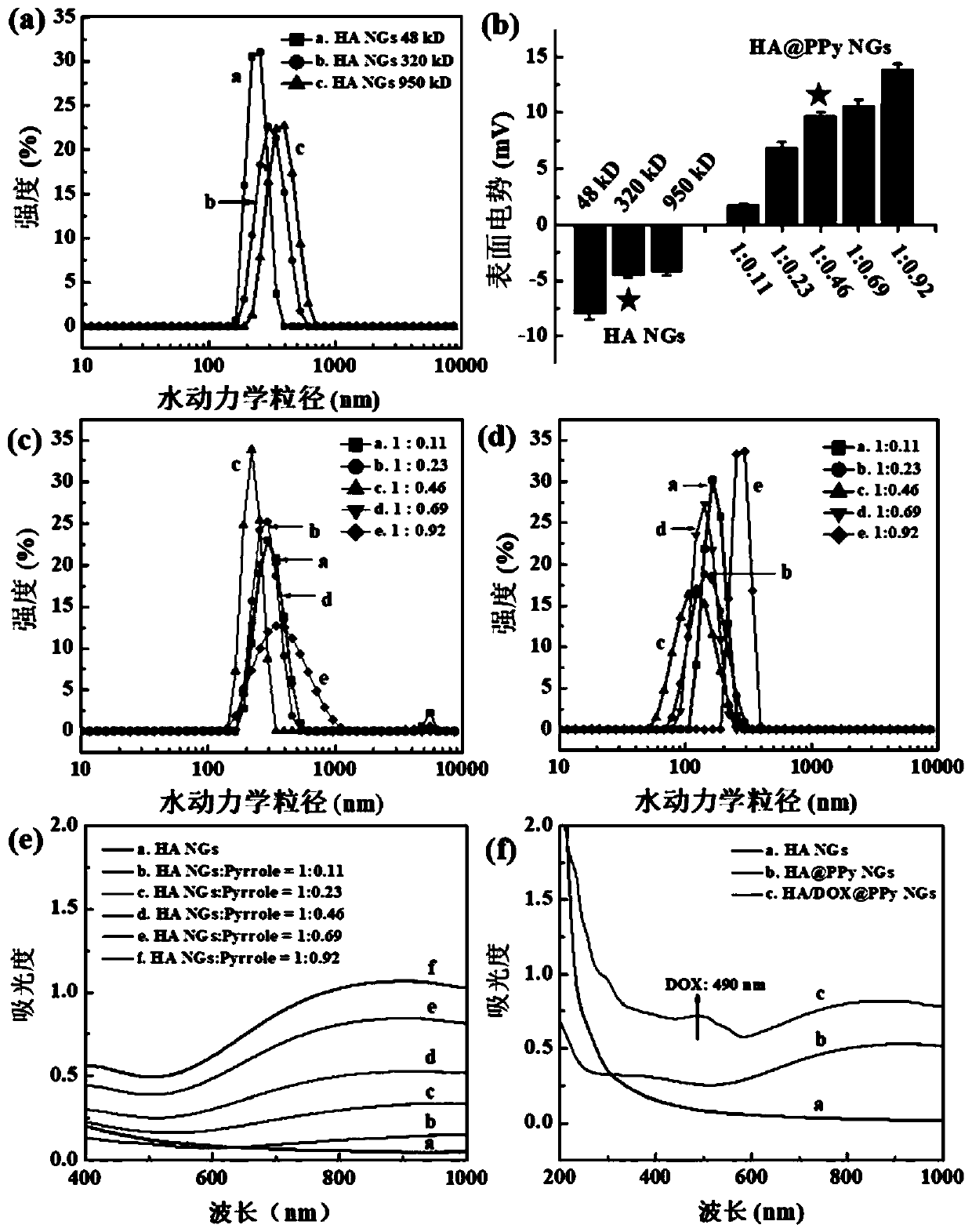
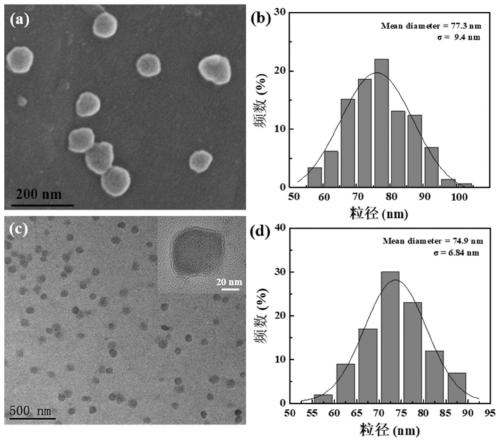
[0070] The HA NGs, HA@PPy NGs and HA / DOX@PPy NGs prepared in Example 1 were characterized. The hydrodynamic particle size distribution and potential changes of HA NGs prepared from hyaluronic acid with different molecular weights are as follows: figure 2 As shown in a and 2b, as the molecular weight of HA increases (48, 320, 950kDa), the hydrodynamic particle size of the prepared HA NGs increases from 243.77nm to 316.67nm and 383.0nm respectively, and the surface potential also increases slightly . However, in the process of preparing HA NGs, it was found that when the molecular weight of HA reaches 950kDa, due to its high viscosity, demulsification is prone to occur during the W / O / W emulsification process. Therefore, 48kDa and 320kDa HA NGs were selected for subsequent research on PPy loading. The results are as follows: figure 2 c, 2d and Table 1, with the increase of the mass ratio of pyrrole monomer Py (HA:Py=1:0.11,1:0.23,1:0.46,1:0.69,1:0.92), the prepared HA@PPy NGs...
Embodiment 3
[0078] The drug release results of drug-loaded nanogel HA / DOX@PPy NGs (350kDa, HA:Py=1:0.46) are as follows Figure 5 as shown in a. The citric acid buffer solution with pH=5.0 and the phosphate buffer solution with pH=7.4 were respectively selected as sustained-release media to investigate the release performance of DOX in different pH environments. First, weigh 1 mg HA / DOX@PPy NGs and dissolve them in 1 mL of the corresponding buffer solution, place them in cellulose membrane dialysis bags with MWCO=14000, and suspend them in 9 mL of PBS solution (pH= 7.4) or citric acid buffer solution (pH=5.0) in a 50mL centrifuge tube, the total volume of the solution in the centrifuge tube is 10mL, each sample has 3 parallels, and then placed in a constant temperature shaker at 37°C for shaking. At the set time point, take 1 mL of sustained-release solution in the EP tube, measure its UV-Vis absorption value at 490 nm, and add 1 mL of new buffer solution at the same time to keep the tot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| photothermal conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com