Boron-doped mesoporous flower-like ferroferric oxide/carbon composite wave-absorbing material and preparation method thereof
A technology of iron tetroxide and wave absorbing material, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of dielectric loss, difficult to achieve electromagnetic parameter matching, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Disperse 1 mmol of anhydrous ferric chloride, 1 mmol of CTAB, 2 mmol of boric acid, and 9 mmol of urea in 40 mL of ethylene glycol, and stir magnetically for 4 hours;
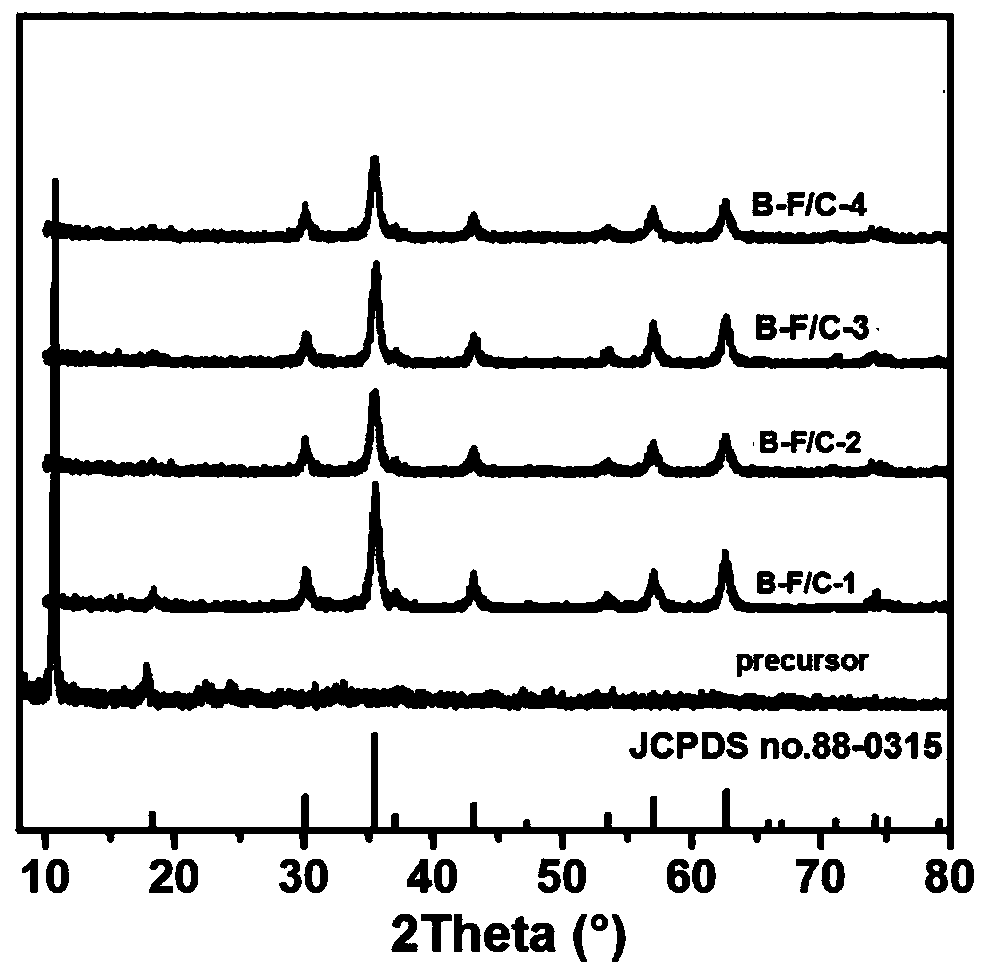
[0034] (2) Transfer the above solution to a high-pressure reactor, put it in an oven at 160°C, take it out after 16 hours, and cool it down to room temperature naturally. Suction filtration, washing with water and absolute ethanol three times each, and oven drying at 60°C for 12 hours to obtain the precursor iron alkoxide, XRD shows figure 1 Shown; XRD results show that the phase of the precursor is iron alkoxide.
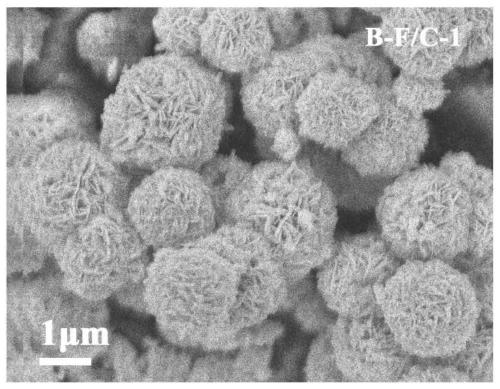
[0035] (3) Grinding the iron alkoxide obtained in step (2) evenly, and calcining at 500° C. for 2 hours in an argon atmosphere. Obtain the composite absorbing material, marked as: B-F / C-1, XRD such as figure 1 Shown; XRD results show that after calcining by argon, the phase of the iron alkoxide of the precursor disappears, and the product of ferric oxide is obtained. SEM such as fi...
Embodiment 2
[0039] (1) Disperse 2mmol of anhydrous ferric chloride, 1mmol of CTAB, 2mmol of boric acid, and 9mmol of urea in 40mL of ethylene glycol, and stir magnetically for 0.5h;
[0040] (2) Transfer the above solution to a high-pressure reactor, put it in an oven at 160°C, take it out after 16 hours, and cool it down to room temperature naturally. The precursor was washed three times with water and absolute ethanol, and dried in an oven at 60°C for 12 hours to obtain the precursor iron alkoxide;
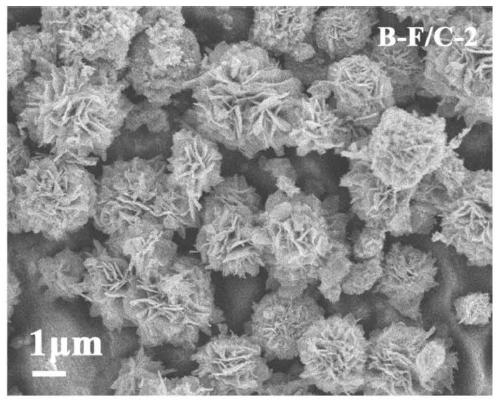
[0041] (3) Grinding the iron alkoxide obtained in step (2) evenly, and calcining at 500° C. for 2 hours in an argon atmosphere. Obtain the composite absorbing material, marked as: B-F / C-2, XRD such as figure 1 As shown, the XRD results show that the composite material obtained by argon calcination is ferric oxide. SEM such as image 3 As shown, the SEM results show that the morphology of the composite material presents an obvious flower-like morphology with a diameter of 2-3 μm. Compar...
Embodiment 3
[0045] (1) Disperse 3mmol of anhydrous ferric chloride, 1mmol of CTAB, 2mmol of boric acid, and 9mmol of urea in 40mL of ethylene glycol, and stir magnetically for 0.5h;
[0046] (2) Transfer the above solution to a high-pressure reactor, put it in an oven at 160°C, take it out after 16 hours, and cool it down to room temperature naturally. The precursor was washed three times with water and absolute ethanol, and dried in an oven at 60°C for 12 hours to obtain the precursor iron alkoxide;
[0047] (3) Grinding the iron alkoxide obtained in step (2) evenly, and calcining at 500° C. for 2 hours in an argon atmosphere. Obtain the composite absorbing material, marked as: B-F / C-3, XRD such as figure 1 As shown, the XRD results show that the composite material obtained by calcination is ferric oxide. SEM such as Figure 4 As shown, the composite material presents a flower-like structure with a diameter of more than 3 μm, and compared with Examples 1 and 2, the flower-like struct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com