Copper coordination compound with property of catalyzing photodegradation of dyes, and preparation method thereof
A copper complex, photodegradation technology, applied in the direction of organic chemistry methods, copper organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., to achieve good removal effect, easy operation and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1 ligand BMIOPE:
[0025] 4,4'-bis(2-methyl -1-imidazolyl) diphenyl ether (BMIOPE);
[0026]
[0027] The molar ratio of 4,4'-dibromodiphenyl ether: 2-methylimidazole: potassium carbonate: cuprous oxide is 2:6:8:1; the reaction temperature is 150° C., and the reaction time is 3 days.
Embodiment 2
[0028] The synthesis of embodiment 2 complexes:
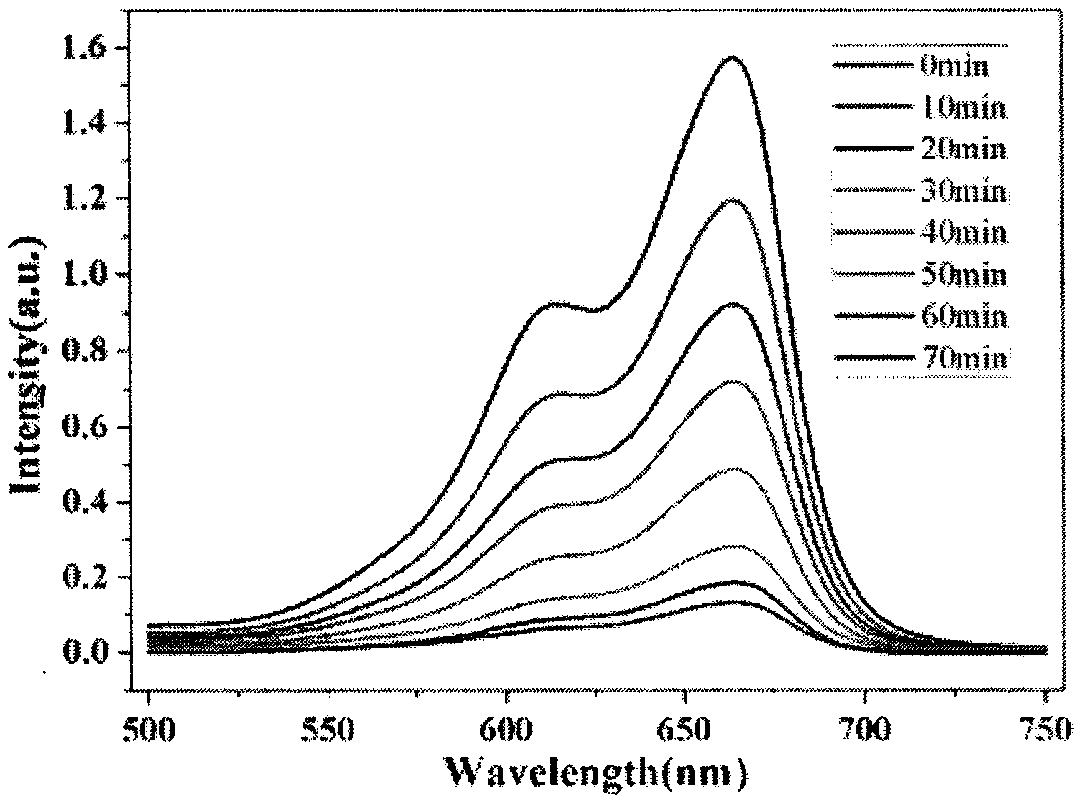
[0029] 12.08mg of copper nitrate trihydrate (Cu(NO 3 ) 2 ·3H 2 O), 16.5 mg of 4,4'-bis(2-methyl-1-imidazolyl) diphenyl ether (BMIOPE), 12.3 mg of 5-bromoisophthalic acid (H 2 TBIP) was dissolved in 0.5mL dimethylformamide (DMF) and 2.5mL aqueous solution, ultrasonically oscillated for 5 minutes to mix evenly, and after mixing, put it into the polytetrafluoroethylene liner of the hydrothermal reaction kettle to obtain a mixed solution, and the above The mixed solution was baked at 100°C for 72 hours, and the solid was separated after the product was taken out to obtain blue blocky crystals.
Embodiment 3
[0030] The structural characterization of embodiment 3 complexes:
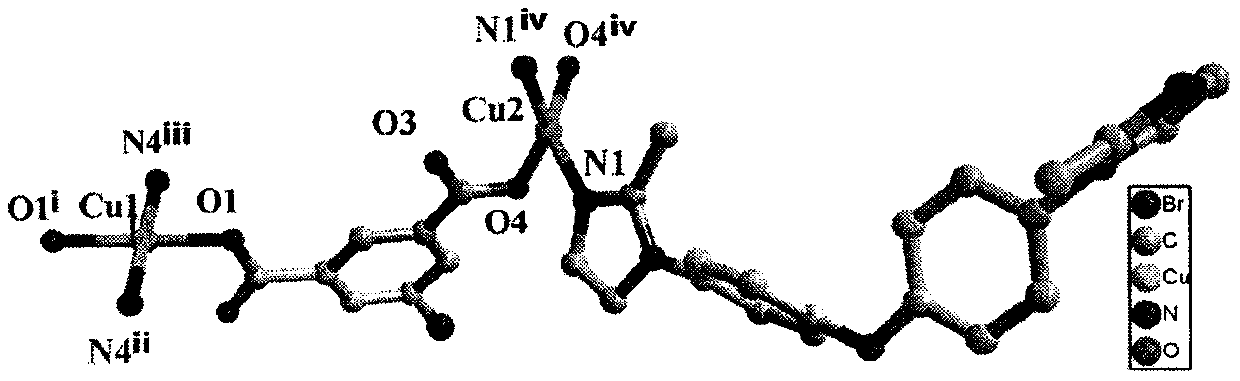
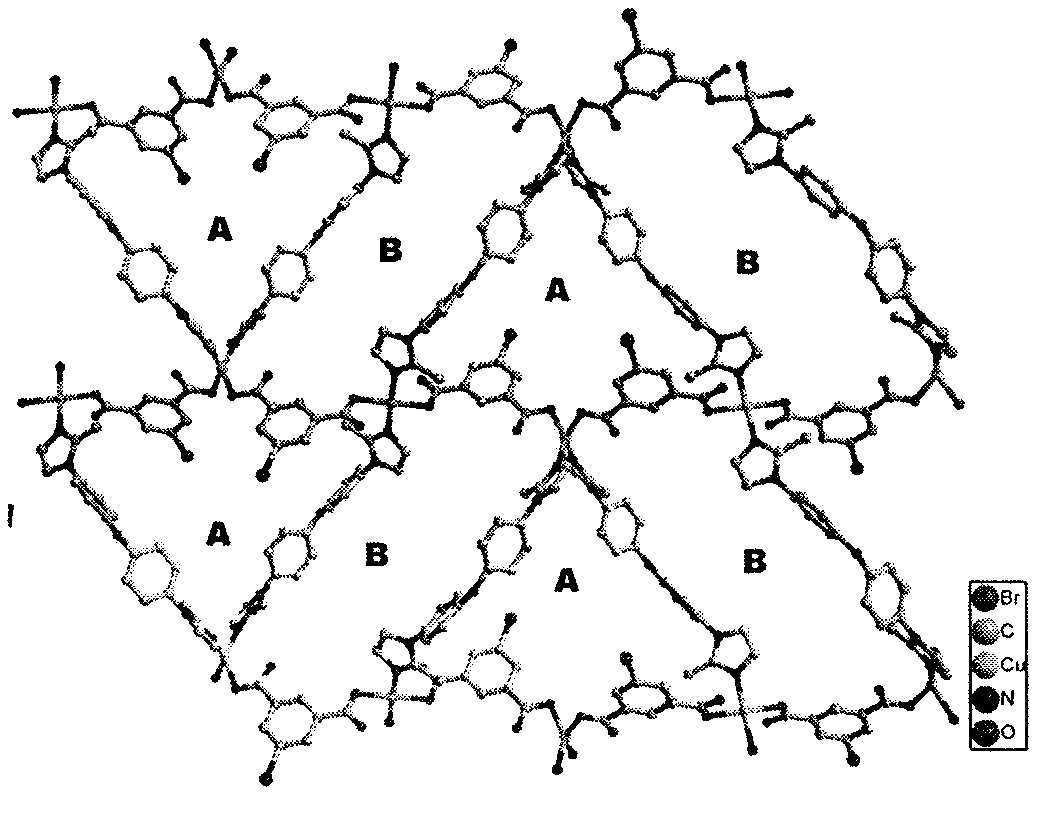
[0031] Use a microscope to select a single crystal of a suitable size, and use a Siemens (Bruker) SMART CCD diffractometer (graphite monochromator, Mo-Ka, ) to collect diffraction data. Diffraction data were corrected for absorption using the SADABS program. Data restoration and structure elucidation were done using the SAINT and SHELXTL programs, respectively. The coordinates of all non-hydrogen atoms were determined by the least square method, and the positions of the hydrogen atoms were obtained by the theoretical hydrogenation method. The crystal structure was refined using the least squares method. Figure 1(a) and Figure 1(b) show the basic coordination and stacking modes. Some parameters of its crystallographic diffraction point data collection and structure refinement are shown in the table below.
[0032] Table 1 Crystallographic data of the complexes
[0033]
[0034]
[0035] R 1 =∑||F ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com