Erythrocyte gel delivery system as well as preparation method and application thereof
A technology for red blood cells and gels, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., to achieve high drug loading, high biosafety and biodegradability, and a preparation method. Simple and fast effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Synthesis and characterization of erythrocyte gels
[0066] (1) Take out 200 μL of blood rapidly from the vein of the organism;
[0067] (2) Immediately inject the fresh blood obtained in step (1) into a sterile mold, and place it at room temperature for 10 minutes;
[0068] (3) Transfer the preliminarily coagulated blood in step (2) to a sterile vacuum drying oven, and dry it gently at 37°C to obtain red blood cell gel, see figure 1 ;
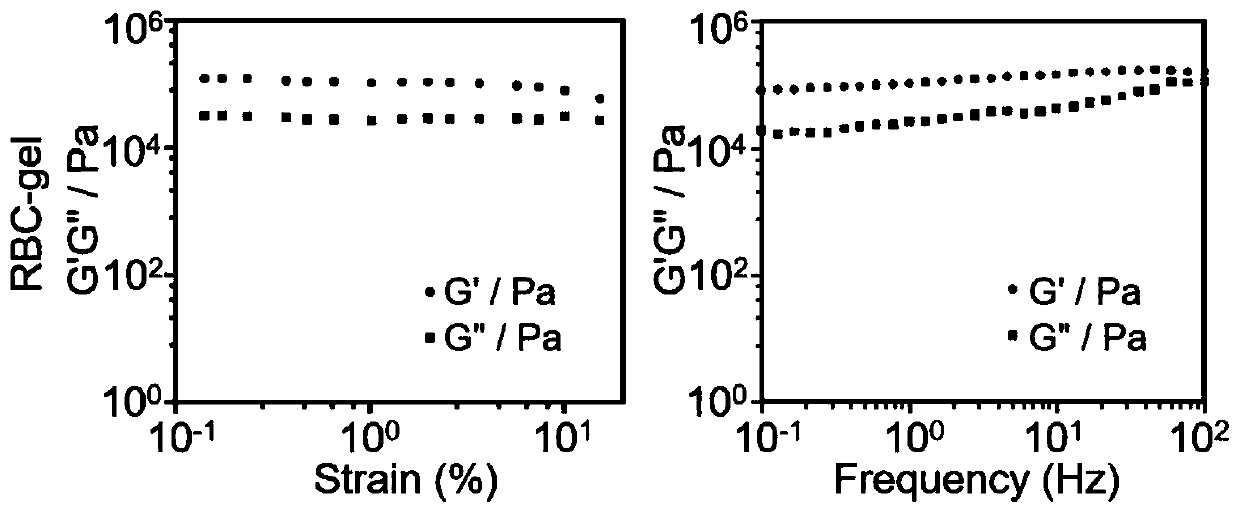
[0069] (4) Use a rheometer to prove the rheological properties of the gel obtained in step (3), which proves that the erythrocyte gel of the present invention has good mechanical properties ( figure 2 );
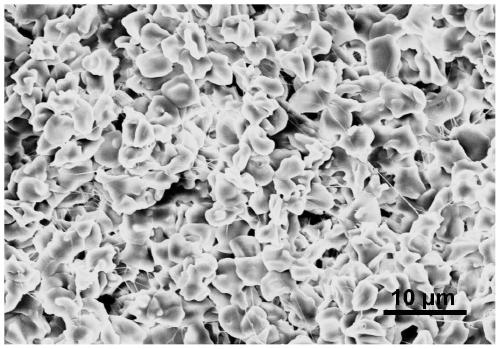
[0070] (5) Using a scanning electron microscope (Scanning Electron Microscope, SEM) to perform microscopic imaging of the erythrocyte gel ( image 3 ), proving that the erythrocyte gel has a regular internal structure;
[0071] (6) Record the degradation of the erythrocyte gel carrier in vivo and in vitro, Figure 4 ...
Embodiment 2
[0073] Example 2: Immunostimulation and immune cell recruitment of erythrocyte gel
[0074] (1) After C57BL / 6J mice were anesthetized with 2.5% isoflurane, the erythrocyte gel prepared in Example (1) was implanted subcutaneously in the mice;
[0075] (2) The erythrocyte gels implanted in the body at 0, 1, 3, and 7 days were taken out at the same time, embedded in paraffin for HE tissue section and embedded in OCT gel for fluorescence staining analysis. It can be seen that the erythrocyte gel of the present invention has the function of recruiting immune cells ( Figure 10 ), a large number of white blood cells infiltrated from the edge of the red blood cell gel to the inside ( Figure 11 ), the fluorescent signal of white blood cells gradually increased from the edge of the red blood cell gel to the inside ( Figure 12 ), the overall fluorescence intensity increases with time ( Figure 13 );
[0076] (3) Further use flow cytometry to analyze the immune cells and their subp...
Embodiment 3
[0077] Example 3: Analysis of drug sustained release effect of erythrocyte gel
[0078] (1) Put Cy5.5-CpG-OND and FITC-OVA into the corresponding molds of step (2) of Example 1 respectively, mix with fresh isolated blood according to the steps in Example 1, and dry gently to prepare drug-loaded red blood cell gel;
[0079] (2) Aspirate a small amount of liquid precipitated from the drug-loaded erythrocyte gel, detect the unloaded drug content with a microplate reader, and calculate the drug-loading efficiency of the erythrocyte gel. The efficiencies of OVA and CpG loaded on erythrocyte gel can reach 95% and 83% ( Figure 15 );
[0080] (3) The prepared drug-loaded erythrocyte gel was embedded in OCT gel, and after sectioning, the drug-loading situation was observed through a confocal microscope. The results showed that OVA could be effectively loaded into the erythrocyte gel ( Figure 16 );
[0081] (4) Place the drug-loaded erythrocyte gel in 2 mL of 37°C Phosphate Buffer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com