A class of aza-condensed and conjugated ladder polymers and their preparation methods and applications in catalytic water splitting under visible light
A polymer and visible light technology, applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of increased cost, complicated operation, unfavorable photocatalytic industrial applications, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
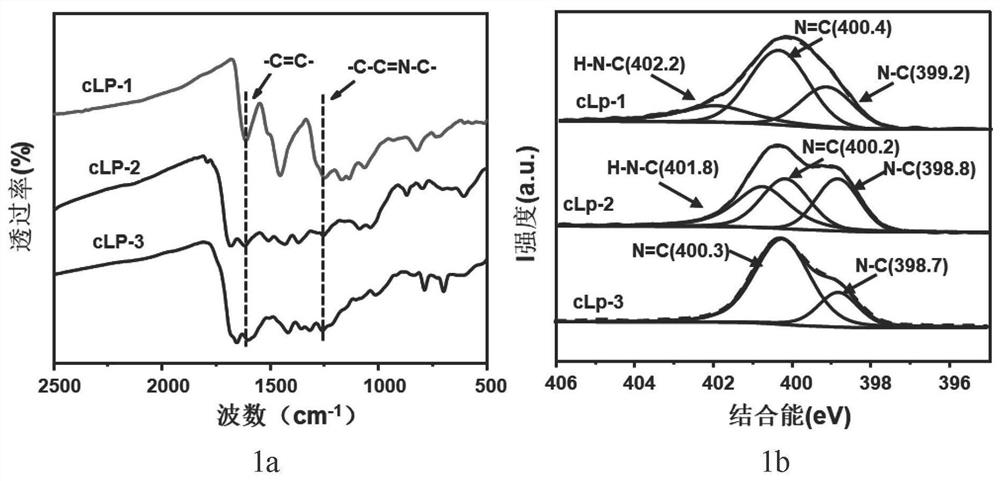
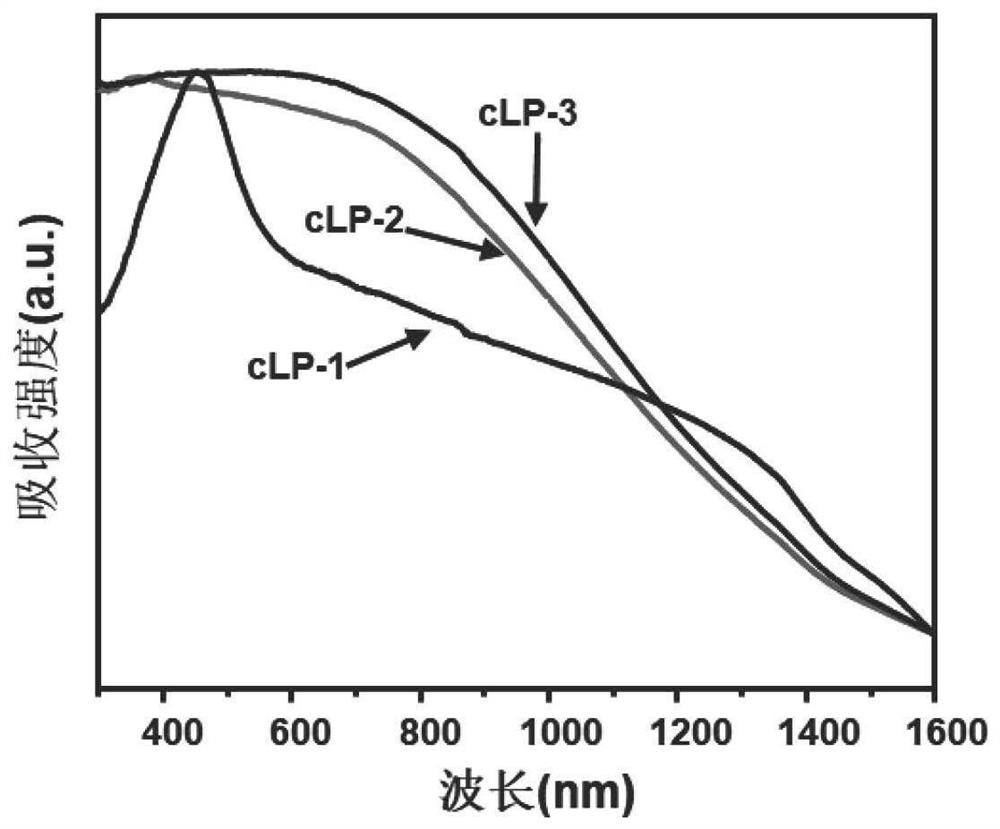
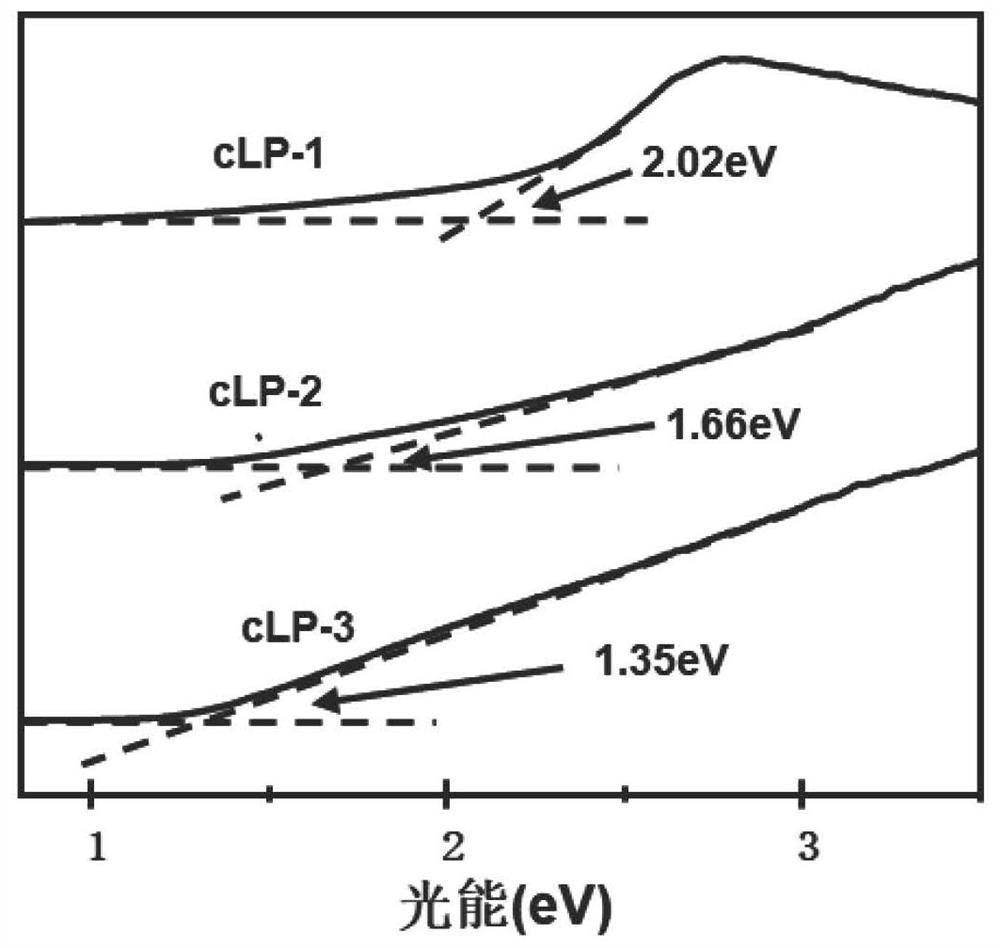
[0075] Add 100 grams of polyphosphoric acid (PAA) to a 150 ml two-neck flask equipped with a nitrogen inlet and outlet, control the nitrogen flow rate to be 30 ml / min to blow air into the polyphosphoric acid, and heat the polyphosphoric acid at 120 ° C 24 hours to completely deoxidize polyphosphoric acid. Then the temperature of the system was lowered to 50°C, and 568 mg of 1,2,4,5-benzenetetramine tetrahydrochloride was quickly added to the deoxygenated polyphosphoric acid in a nitrogen atmosphere, while the system was heated to 120°C for 12 hours To remove all the hydrogen chloride in 1,2,4,5-benzenetetramine tetrahydrochloride. Then, 282 mg of 2,5-dihydroxy-1,4-benzoquinone was added to the mixture at 110°C, and the mixture was slowly heated (with a heating rate of 4°C / min) to 180°C and kept for 12 hours. After cooling the obtained viscous solution to room temperature, transfer it to a 500 ml beaker, and add 400 ml of water to the beaker, stir vigorously so that polyphosph...
Embodiment 2
[0078] Add 1 g of 1,2,4,5-benzenetetramine tetrahydrochloride and 500 mg of piperazine-2,3,5,6-tetraketone into a 200-ml round-bottomed flask under an inert atmosphere, and then place the round-bottomed The flask was placed in an ice bath (temperature controlled at 0°C). 80 milliliters of N-methylpyrrolidone (NMP) was deoxidized, then mixed with 0.5 milliliters of sulfuric acid, and the mixed solution was slowly added dropwise to the above-mentioned round bottom flask. Then the reaction device was raised to room temperature, and after stirring for 2 hours, an oil bath was used instead of an ice-water bath and the entire reaction device was heated to 180°C. After continuous reaction for 8 hours, the heating was stopped and the device was cooled to room temperature. Add water to quench the reaction in the round-bottomed flask, and the suspension after the reaction uses polytetrafluoroethylene (PTFE, 0.22 micron) organic filter membrane to carry out suction filtration to obtain t...
Embodiment 3
[0081] Add 1 g of 1,2,4,5-benzenetetramine tetrahydrochloride and 922 mg of pyrene-4,5,9,10-tetraketone into a 200-ml round-bottomed flask under an inert atmosphere, and then place the round-bottomed flask Place in an ice bath (temperature controlled at 0°C). 80 milliliters of N-methylpyrrolidone (NMP) was deoxidized, then mixed with 0.5 milliliters of sulfuric acid, and the mixed solution was slowly added dropwise to the above-mentioned round bottom flask. Then the reaction device was raised to room temperature, and after stirring for 2 hours, an oil bath was used instead of an ice-water bath and the entire reaction device was heated to 180°C. After continuous reaction for 8 hours, the heating was stopped and the device was cooled to room temperature. Add water to the round-bottomed flask to quench the reaction, and the suspension after the reaction is suction-filtered with a polytetrafluoroethylene (PTFE, 0.22 micron) organic filter membrane to obtain a black solid product, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com