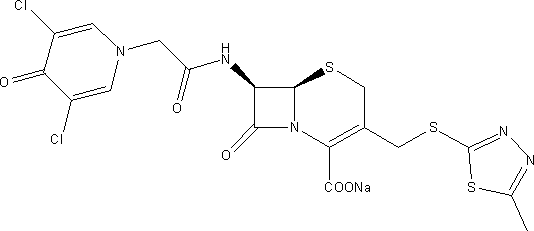
Synthesis process of cefazedone sodium
A synthesis process and technology of cephalosporins, which are applied in the field of pharmaceutical synthesis, can solve the problems of being unsuitable for industrialized large-scale production, difficult to find purification methods, and high industrial production costs, and achieve the advantages of reducing the occurrence of by-products, reducing product costs, and reducing side reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of Thioester Compounds
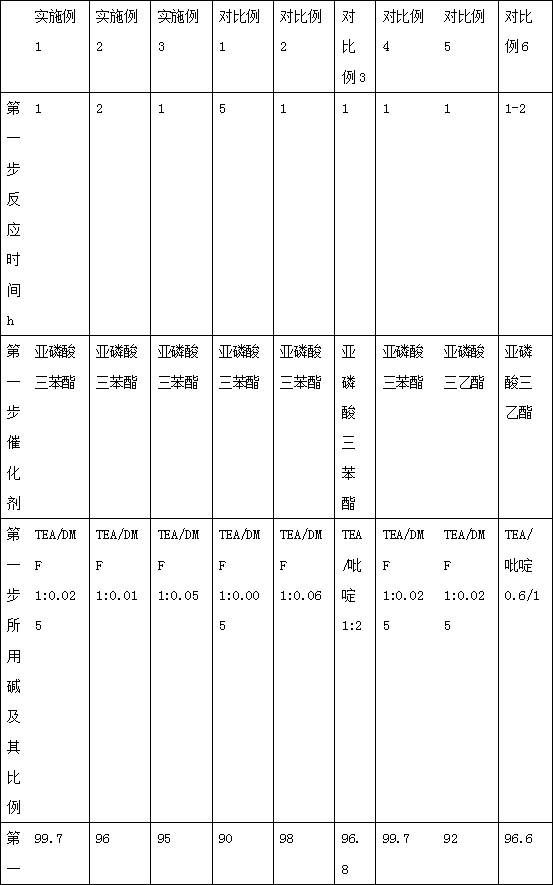
[0031] In a dry reaction flask, add 200ml of dichloromethane, 22.2g (0.1mol) of 3,5-dichloro-4-pyridone-1-acetic acid and 2-mercapto-5-methyl-1,3,4 -Thiadiazole 14.55g (0.11mol), stirred, then added triethylamine 10.1g (0.1mol), DMF 1.83g (0.025mmol) stirred at room temperature for 1 hour, then slowly added dropwise the catalyst triphenyl phosphite Dichloromethane solution (31g of triphenyl phosphite dissolved in 50ml of dichloromethane, 0.1mol), dripped within 1 hour, controlled the temperature at 20-25°C, stirred and reacted for 1 hour, cooled to below 10°C, filtered, and the filtrate Spin dry, and vacuum dry below 40°C to obtain 33.5 g (0.099 mol) of the thioester compound, with a yield of 99.7% and an HPLC purity of 99.5%.
[0032] (2) Preparation of Cefazedone Sodium:
[0033] Under the protection of nitrogen, add 24.5g (0.09mol) of 7-ACA and 3.6g (0.09mol) of sodium hydroxide into the mixed solvent of water and acetonitri...
Embodiment 2
[0035] (1) Preparation of Thioester Compounds
[0036]In a dry reaction flask, add 200ml of dichloromethane, 22.2g (0.1mol) of 3,5-dichloro-4-pyridone-1-acetic acid and 2-mercapto-5-methyl-1,3,4 -Thiadiazole 14.55g (0.11mol), stirred, then added triethylamine 10.1g (0.1mol), DMF 0.73g (0.01mmol) stirred at room temperature for 1 hour, then slowly added dropwise the catalyst triphenyl phosphite Dichloromethane solution (31g of triphenyl phosphite dissolved in 50ml of dichloromethane, 0.1mol), dripped within 1 hour, stirred and reacted at 20-25°C for 2 hours, cooled to below 10°C, filtered, The filtrate was spin-dried and vacuum-dried below 40°C to obtain 32.3 g (0.096 mol) of the thioester compound with a yield of 96% and an HPLC purity of 99.5%.
[0037] (2) Preparation of Cefazedone Sodium:
[0038] Under the protection of nitrogen, add 24.5g (0.09mol) of 7-ACA and 3.6g (0.09mol) of sodium hydroxide into the mixed solvent of water and acetonitrile (100ml of water and 200ml ...
Embodiment 3
[0040] (1) Preparation of Thioester Compounds
[0041] In a dry reaction flask, add 200ml of dichloromethane, 22.2g (0.1mol) of 3,5-dichloro-4-pyridone-1-acetic acid and 2-mercapto-5-methyl-1,3,4 -Thiadiazole 14.55g (0.11mol), stirred, then added triethylamine 10.1g (0.1mol), DMF 3.65g (0.05mmol) stirred at room temperature for 1 hour, then slowly added dropwise the catalyst triphenyl phosphite Dichloromethane solution (31g of triphenyl phosphite dissolved in 50ml of dichloromethane, 0.1mol), dripped within 1 hour, controlled the temperature at 20-25°C, stirred and reacted for 1 hour, cooled to below 10°C, filtered, and the filtrate Spin dry, and vacuum dry below 40°C to obtain 31.9 g (0.095 mol) of the thioester compound, with a yield of 95% and an HPLC purity of 99.5%.
[0042] (2) Preparation of Cefazedone Sodium:
[0043] Under the protection of nitrogen, add 24.5g (0.09mol) of 7-ACA and 3.6g (0.09mol) of sodium hydroxide into the mixed solvent of water and acetonitrile ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com