Fusion protein of MNK2 protein kinase and cell-penetrating peptide, hydrogel of fusion protein, and application of fusion protein in promoting myocardial regeneration
A fusion protein and protein kinase technology, applied in the field of biomedicine, can solve the problem that there is no MNK2 protein kinase to promote myocardial regeneration or treat cardiomyopathy literature reports, etc., and achieve the effect of feasible implementation conditions, simple operation, and delaying ventricular remodeling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
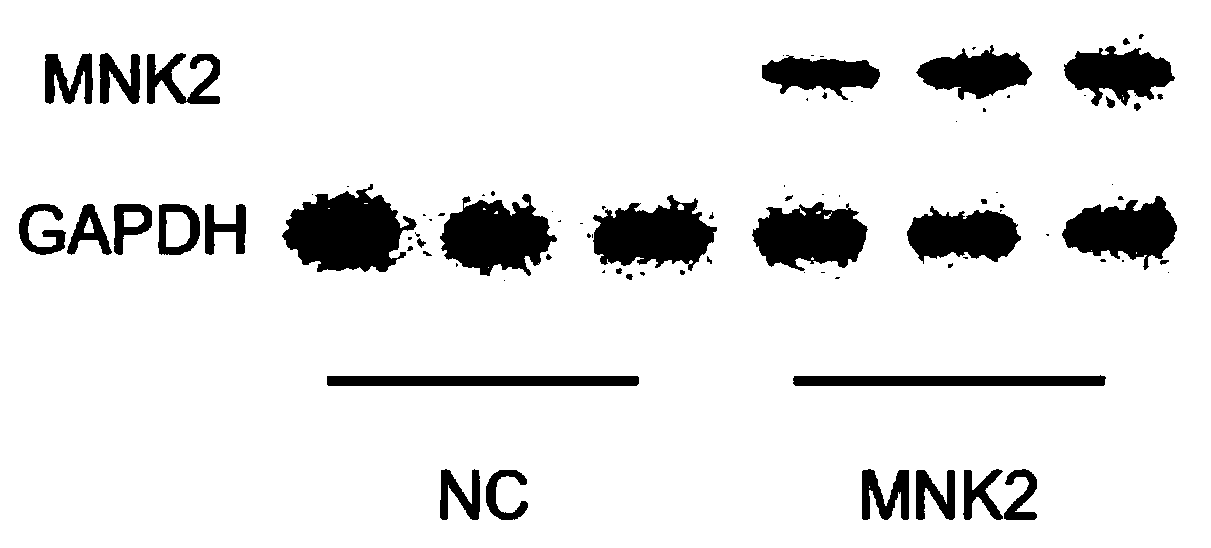
[0032] Example 1 Preparation of biologically active recombinant human MNK2 protein kinase
[0033] 1.> TAT-MNK2 (nucleotide sequence) is shown in SEQ NO.3.
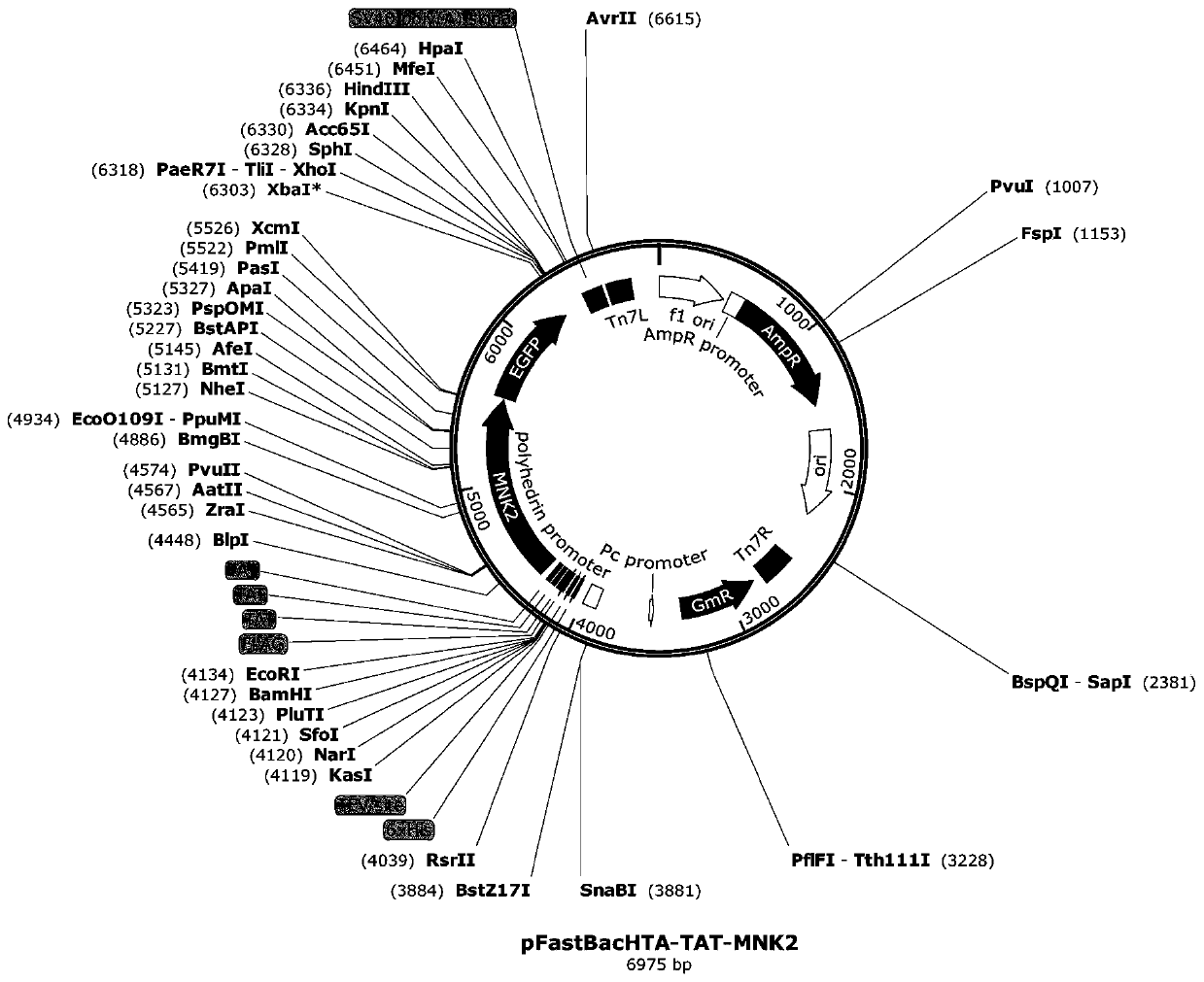
[0034] 2. Construction of pFastBacHTA-MNK2 insect cell vector
[0035]The Bac-to-BacHTVectorKit is designed for expression and purification of histidine-tagged recombinant proteins in Sf9, Sf21 or HighFive cells after baculovirus vector generation in Escherichia coli (E.coli). The pFastBacHT vector has the following features: (1) strong polyhedrin promoter for protein expression; (2) three reading frames for simplified cloning; (3) N-terminal 6xHis tag for easy purification of recombinant fusion proteins (4) TEV protease cleavage site for removal of his-tag after protein purification.
[0036] Construction process of recombinant vector
[0037] (1) Plasmid design:
[0038] pUCori, F1ori terminator: SV40poly(A) signal; restriction site 5'BamHI, 3'HindIII; TAT-MNK2 primer design requirements: GC value between 40-55 is m...
Embodiment 2
[0128] Embodiment 2 Preparation of recombinant human MNK2 protein kinase hydrogel
[0129] (1) Weigh 20mg of colloidal precursor molecular powder and fully dissolve it with 800uL double distilled water at room temperature. The solution was sterilized by filtration with a 0.22um bacteria filter, the pH was adjusted to 7.4 with sterile sodium bicarbonate powder, and the total volume of the solution was added to 1000ul (hereinafter referred to as solution A).
[0130] (2) Take out the prepared recombinant human MNK2 protein kinase solution of known concentration from the -80°C refrigerator and dissolve it on ice, take the solution containing 10 mg of protein kinase, and use sterile PBS to make up the total volume of the protein solution to 1000ul ( Hereinafter referred to as liquid B)
[0131] (3) Mix 1000 ul of liquid A and liquid B at room temperature, and gently blow and mix with a pipette to obtain the small molecule hydrogel-recombinant human MNK2 protein kinase mixture, wh...
Embodiment 3
[0132] Example 3 Application of recombinant human MNK2 protein kinase hydrogel in promoting myocardial regeneration and repair
[0133] 1. Preparation and administration of mouse model of acute myocardial infarction
[0134] Fifty 8-week-old (P56) male mice were anesthetized by intraperitoneal injection ( / kg) of 1.2070 Avertin, and artificially ventilated with a small animal ventilator after tracheal intubation. Use ophthalmic scissors to cut the skin along the left fourth intercostal space. Ophthalmic forceps bluntly separate the intercostal muscles and enter the chest cavity to expose the left atrial appendage. Use a 6-0 needle from the lower edge of the left atrial appendage, and the anterior wall of the ventricle becomes pale. , The needle is taken out from the junction of the conus pulmonary artery and the left atrial appendage, and the LAD suture is ligated. Observing the left side indicates that the ligation is correct. The operator lifts up the two ends of the ligatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com