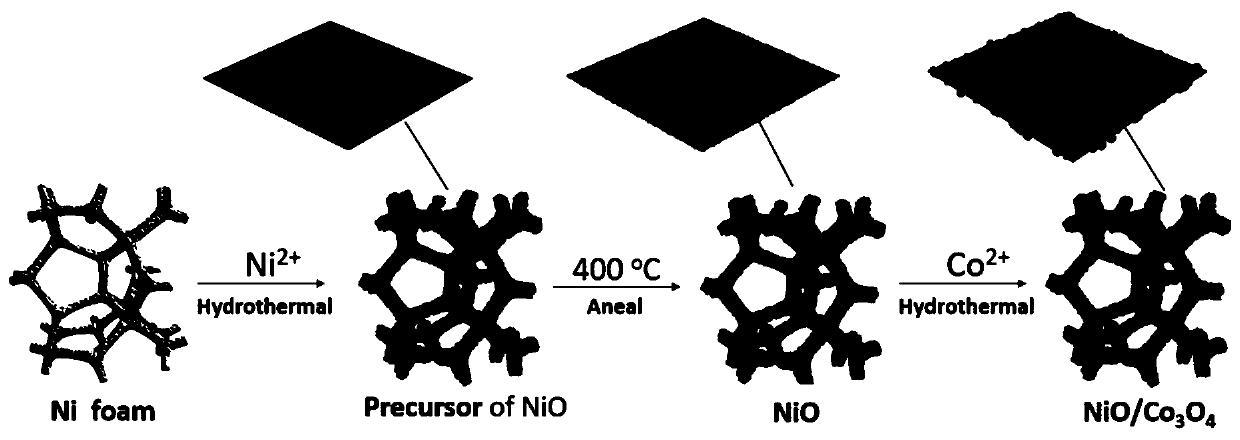
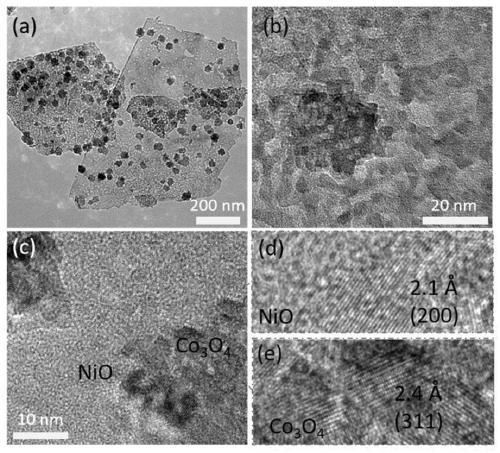
Preparation method and application of NiO/Co3O4
A reaction kettle and hydrothermal reaction technology, which is applied in the field of electrocatalysis and material engineering, can solve the problems of affecting the catalytic efficiency of the catalyst, the contribution of the active site, and the complicated operation, so as to shorten the electron transmission path, simplify the preparation process, and improve the activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A NiO / Co 3 o 4 The preparation method of the composite is to weigh 0.435 g of nickel nitrate hexahydrate, 0.450 g of urea, and 0.111 g of ammonium fluoride, add deionized water and stir at room temperature to prepare a 60 mL transparent solution, and the solution filling ratio in the reactor is 60vol. %, the concentrations of nickel nitrate hexahydrate, ammonium fluoride, and urea in the solution can be calculated to be 25 mmol / L, 50 mmol / L, and 125 mmol / L, respectively. The solution was then transferred to a polytetrafluoroethylene substrate in a 100 mL reactor, and the filling ratio of the solution was 60 vol.%. Add the cleaned 2×2 cm foam nickel substrate, tighten the stainless steel metal cover of the reaction kettle, and place it in a blast drying oven for hydrothermal reaction. The hydrothermal temperature is 110 °C and the time is 10 h. After cooling to room temperature, the nickel foam was taken out, rinsed with deionized water and alcohol, and then dried at ...
Embodiment 2
[0032] A NiO / Co 3 o 4 The preparation method of the complex is to weigh nickel nitrate hexahydrate, urea and ammonium fluoride 0.290 g, 0.300 g, and 0.074 g respectively, add deionized water and stir at room temperature to form a 40 mL solution. The filling ratio of the solution in the reactor is 40vol.%, the concentrations of nickel nitrate hexahydrate, ammonium fluoride, and urea in the solution can be calculated to be 25 mmol / L, 50 mmol / L, and 125 mmol / L, respectively. The resulting solution was transferred to a 100 mL reactor with a solution filling ratio of 40 vol.%. And add the cleaned 1×1 cm foam nickel as the growth substrate. Hydrothermal reaction at 90 °C for 36 h. After cooling to room temperature, the nickel foam was taken out, rinsed several times with deionized water and alcohol, and then dried at 60 °C to obtain the precursor of nickel oxide grown on the nickel foam substrate. The precursor was placed in a muffle furnace, kept at 300 °C for 6 h at a heating ...
Embodiment 3
[0034] A NiO / Co 3 o 4 The preparation method of the composite is to weigh 0.580 g of nickel nitrate hexahydrate, 0.600 g of urea, and 0.148 g of ammonium fluoride, and add deionized water to prepare 80 mL of uniform and transparent green solution. The filling ratio of the solution in the reactor is 80vol.%. Similarly, the concentrations of nickel nitrate hexahydrate, ammonium fluoride, and urea in the solution can be calculated to be 25 mmol / L, 50 mmol / L, and 125 mmol / L, respectively. The solution was then transferred to a 100 mL reactor with a filling ratio of 80 vol.%. And put the cleaned nickel foam base of 2×3 cm in size, and put it in a blast drying oven for hydrothermal reaction. The hydrothermal temperature is 160 °C and the time is 5 h. That is, the precursor of nickel oxide grown on the nickel foam substrate is obtained. The precursor was placed in a muffle furnace, held at 450 °C for 1 h at a heating rate of 10 °C / min, and cooled to room temperature with the furn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com