Normal-temperature cell preserving fluid and cell preparation for injection
A technology of cell preparation and preservation solution, which is applied in the field of medical biology, can solve the problems of not being able to guarantee temperature stability, not suitable for low-temperature storage, and high price of cold chain, so as to achieve good maintenance of cell biological activity, activity and uniformity, The effect of saving economic cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This example provides a method for preparing a cell preservation solution that can be transported at room temperature, specifically adding compound amino acid injection, human serum albumin injection, and vitamin C injection to normal saline (sodium chloride injection) in sequence In the process, mix thoroughly to complete the preparation.
[0038] In this embodiment, the reagents used and the manufacturer information are as follows:
[0039] Reagent name manufacturers Compound Amino Acid Injection Xinjiang Deyuan Biological Engineering Co., Ltd. Human Albumin Injection Chenxin Pharmaceutical Co., Ltd. Vitamin C Injection Henan Runhong Pharmaceutical Co., Ltd. Sodium Chloride Injection Shandong Qidu Pharmaceutical Co., Ltd. Compound Sodium Chloride Injection Shandong Qidu Pharmaceutical Co., Ltd.
[0040] Wherein, among the above-mentioned reagents, the specific components and contents of each reagent are as follows:
...
Embodiment 2
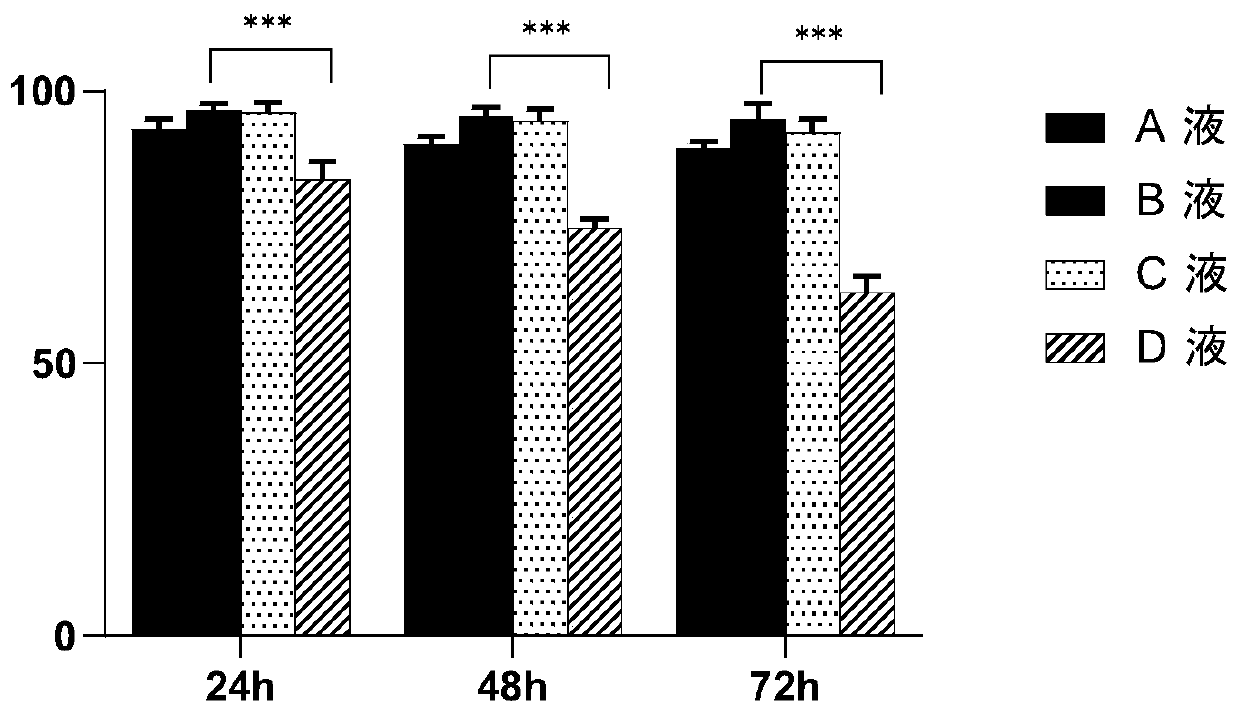
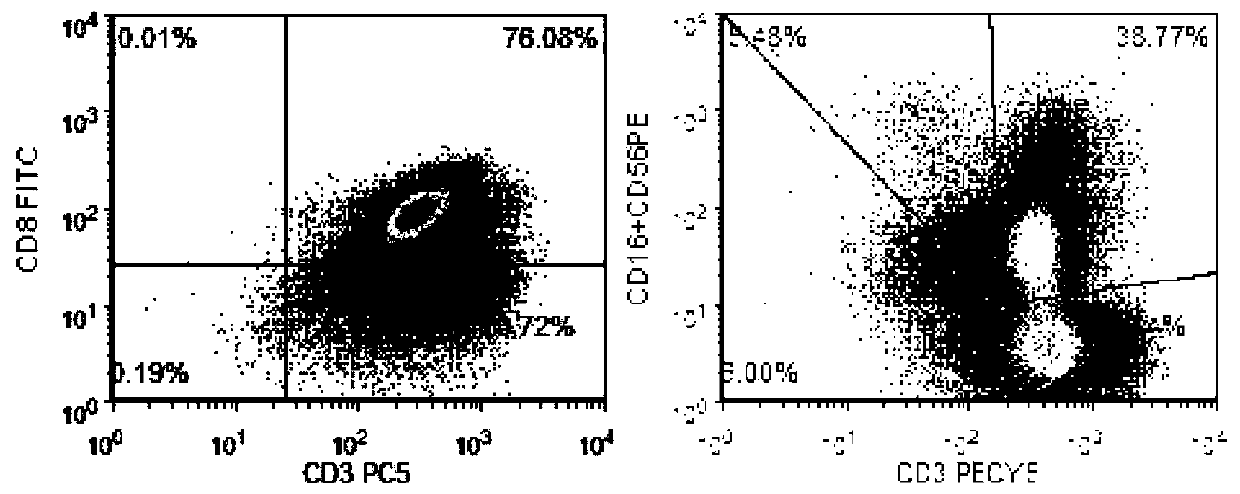
[0067] This example takes immune cells (CIK cells) as an example to verify cell survival rate and activity. The following cell operations are all completed in GMP pharmaceutical-grade laboratories. The specific steps are as follows:
[0068] 1) Take 30-50ml of peripheral blood from the patient, dilute it with PBS solution to an equal volume, perform density gradient centrifugation with Ficoll lymphatic separation medium (2000g, 22°C, 30min), and obtain PBMCS from peripheral blood mononuclear cells, wash them twice with normal saline, and then Suspend the cells with X-VIVO15 basal medium, adjust the cell density to 2×10 6 , placed at 37°C, 5% CO 2 cultured in a carbon dioxide incubator. On the 0th day of culture, add IFN-γ, 1000U / ml, 24h later, add anti-CD3 monoclonal antibody 100ng / ml, human recombinant IL-1α, 100U / ml, human recombinant IL-2, 1000U / ml. Afterwards, the culture medium was replaced every 2 to 3 days and IL-2 was added at 1000 U / ml. Harvest after 14 days.
[0...
Embodiment 3
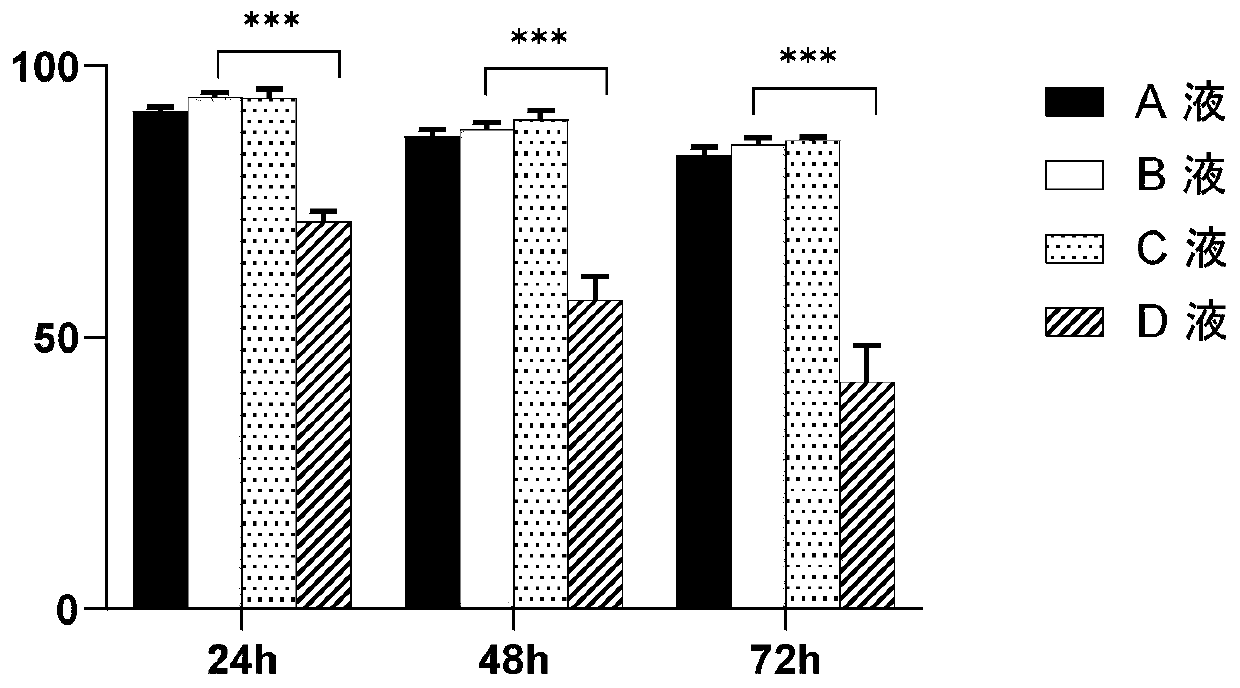
[0079] This example takes umbilical cord mesenchymal stem cells (MSC cells) as an example to verify cell survival and activity. The following cell operations are all completed in GMP pharmaceutical-grade laboratories. The specific steps are as follows:
[0080] 1) Cut off a section of umbilical cord about 10cm long, rinse it repeatedly with normal saline, and cut it to 1-3mm 3 organization block. Use scissors to evenly spread the umbilical cord fragments on a 150mm petri dish, cover the lid, put it in a 37°C incubator upside down and adhere to the wall for 2 hours, then add 20ml of medium (add MSCBM-CD TM Single Quot TM LONZA Mesenchymal Stem Cell Medium with Growth Supplements). Place the Petri dish at 37 °C, 5% CO 2 cultured in an incubator. After culturing for 5-7 days, add 10ml of culture medium. On the 14th day, the cells crawling out were observed, all the tissue pieces were removed, and the cells were subcultured when the cells reached about 80% confluence. After t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com