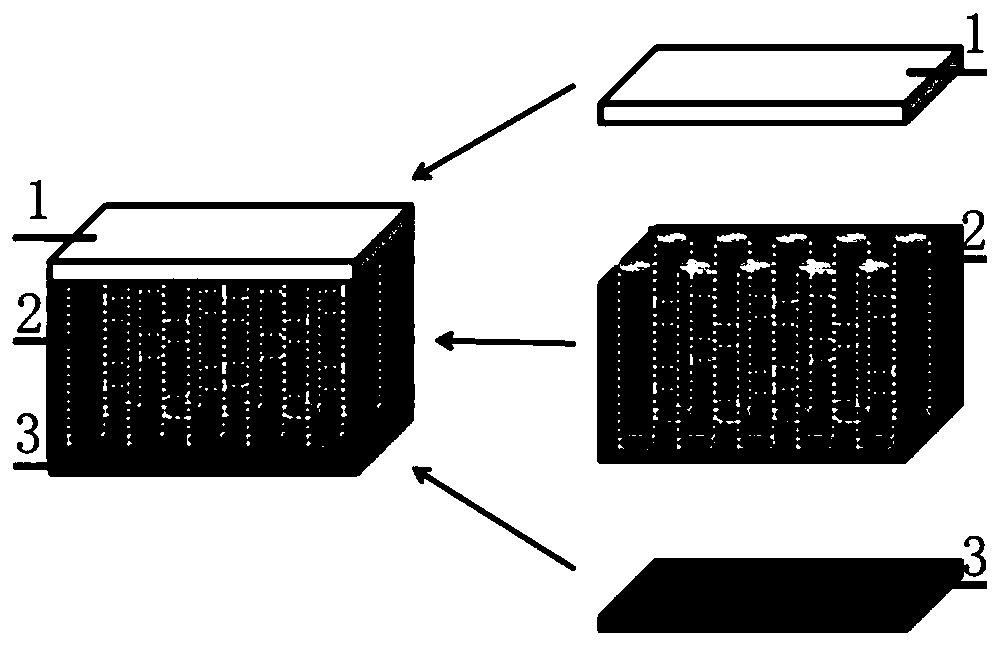
Bionic multilayer collagen support for cartilage repair and preparation method therefor
A collagen scaffold and cartilage repair technology, applied in tissue regeneration, bone implants, medical science, etc., can solve problems such as low effectiveness, low induction of biomaterials, and high risk of immune rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] After pulverizing the stripped tendon, add 10 times the volume of 4M guanidine hydrochloride prepared by pH=7.5 and 0.05M Tris-HCl;
[0081] After suspension, pulverize the homogenate, stir at 4°C for 24 hours, centrifuge at 12000g for 20 minutes, and separate the supernatant and precipitate;
[0082] The precipitate was fully washed with Tris-HCl buffer and 0.5mM acetic acid, and then added acetic acid overnight to remove proteoglycans;
[0083] Dissolve the tendon with pepsin-containing glacial acetic acid solution, continue to process for a period of time until the mixture becomes a colorless, transparent, viscous liquid, centrifuge at 4°C for 20 minutes under 5000g centrifugal force, and collect the supernatant as the crude tendon collagen stock solution;
[0084] Add the sodium chloride solution to it, and keep stirring until white flocculent precipitates are precipitated from the solution, and continue to add the sodium chloride solution until the precipitate is n...
Embodiment 2
[0091] After pulverizing the stripped tendon, add 10 times the volume of 4M guanidine hydrochloride prepared by pH=7.5 and 0.05M Tris-HCl;
[0092] After suspension, pulverize the homogenate, stir at 4°C for 24 hours, centrifuge at 12000g for 20 minutes, and separate the supernatant and precipitate;
[0093] The precipitate was fully washed with Tris-HCl buffer and 0.5mM acetic acid, and then added acetic acid overnight to remove proteoglycans;
[0094] Dissolve the tendon with pepsin-containing glacial acetic acid solution, continue to treat for a period of time until the mixture becomes a colorless, transparent, viscous liquid, centrifuge at 4°C for 20 minutes at 5000g centrifugal force, and collect the supernatant as the crude tendon collagen stock solution;
[0095] Add the sodium chloride solution into it, keep stirring until white flocculent precipitates are precipitated from the solution, and continue to add the sodium chloride solution until the precipitate no longer p...
Embodiment 3
[0102] After pulverizing the stripped tendon, add 10 times the volume of 4M guanidine hydrochloride prepared by pH=7.5 and 0.05M Tris-HCl;
[0103] After suspension, pulverize the homogenate, stir at 4°C for 24 hours, centrifuge at 12000g for 20 minutes, and separate the supernatant and precipitate;
[0104] The precipitate was fully washed with Tris-HCl buffer and 0.5mM acetic acid, and then added acetic acid overnight to remove proteoglycans;
[0105] Dissolve the tendon with pepsin-containing glacial acetic acid solution, continue to treat for a period of time until the mixture becomes a colorless, transparent, viscous liquid, centrifuge at 4°C for 20 minutes at 5000g centrifugal force, and collect the supernatant as the crude tendon collagen stock solution;
[0106]Add the sodium chloride solution to it, and keep stirring until white flocculent precipitates are precipitated from the solution, and continue to add the sodium chloride solution until the precipitate is no long...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com