Coumarin-benzopyranium salt derivative as well as synthesis method and application thereof
A technology of benzopyrylium salt and synthesis method, applied in chemical instruments and methods, instruments, analytical materials and other directions, can solve problems such as death, abnormal metabolism of sulfur dioxide, damage to the brain, nervous system organs, etc., and achieves high sensitivity, Simple detection method and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
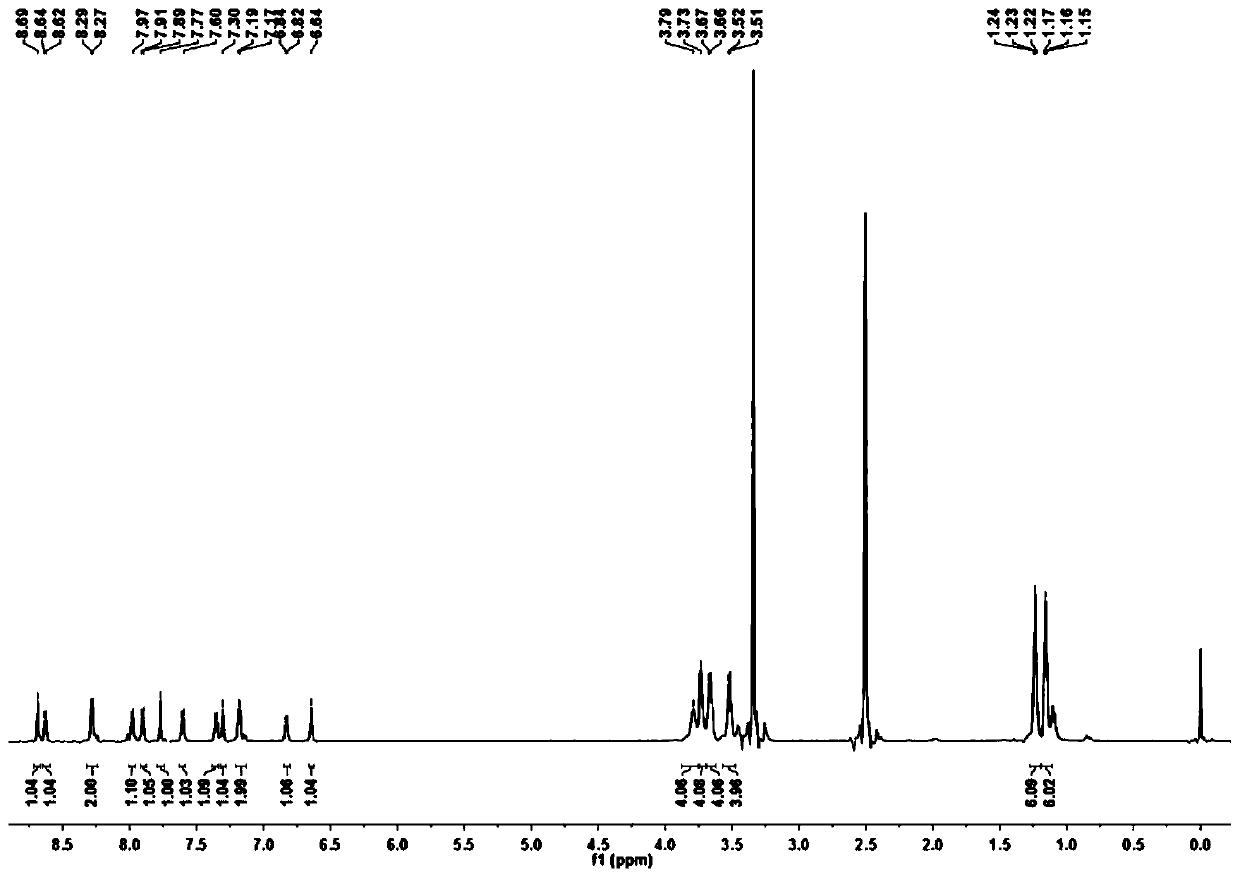
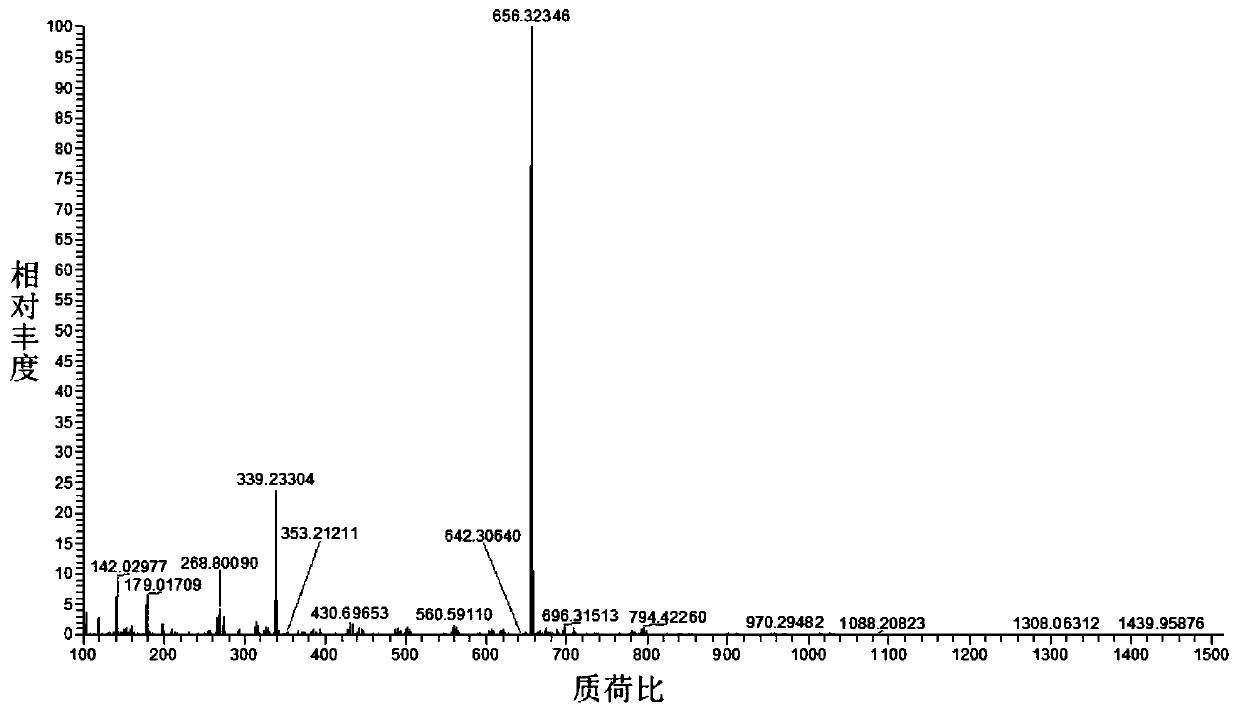
[0048] Preparation and Characterization of Coumarin-benzopyrylium Derivative CM-BP
[0049] (1) Dissolve 4-formyl-7 diethylaminocoumarin (0.49g, 2mmol) and cyanoacetic acid (0.255g, 3mmol) in 100mL of absolute ethanol, and heat the mixture at 87°C for 4 hours; After completion, the solution was spin-dried under reduced pressure, and the obtained orange solid was separated by silica gel column chromatography using methanol and dichloromethane with a volume ratio of 1:15 as the eluent to obtain an orange solid powder (0.303 g, yield: 48.7%);
[0050] (2) 4-diethylamino salicylaldehyde (0.386g, 2mmol) and 4-piperazinyl acetophenone (0.408g, 2mmol) were dissolved in 10mL of concentrated sulfuric acid, the mixture was stirred and refluxed at 90°C for 8 hours, cooled After reaching room temperature, the reaction solution was poured into ice water and continued to stir. The resulting precipitate was filtered, washed, and vacuum-dried to obtain a black solid. The resulting orange soli...
Embodiment 2
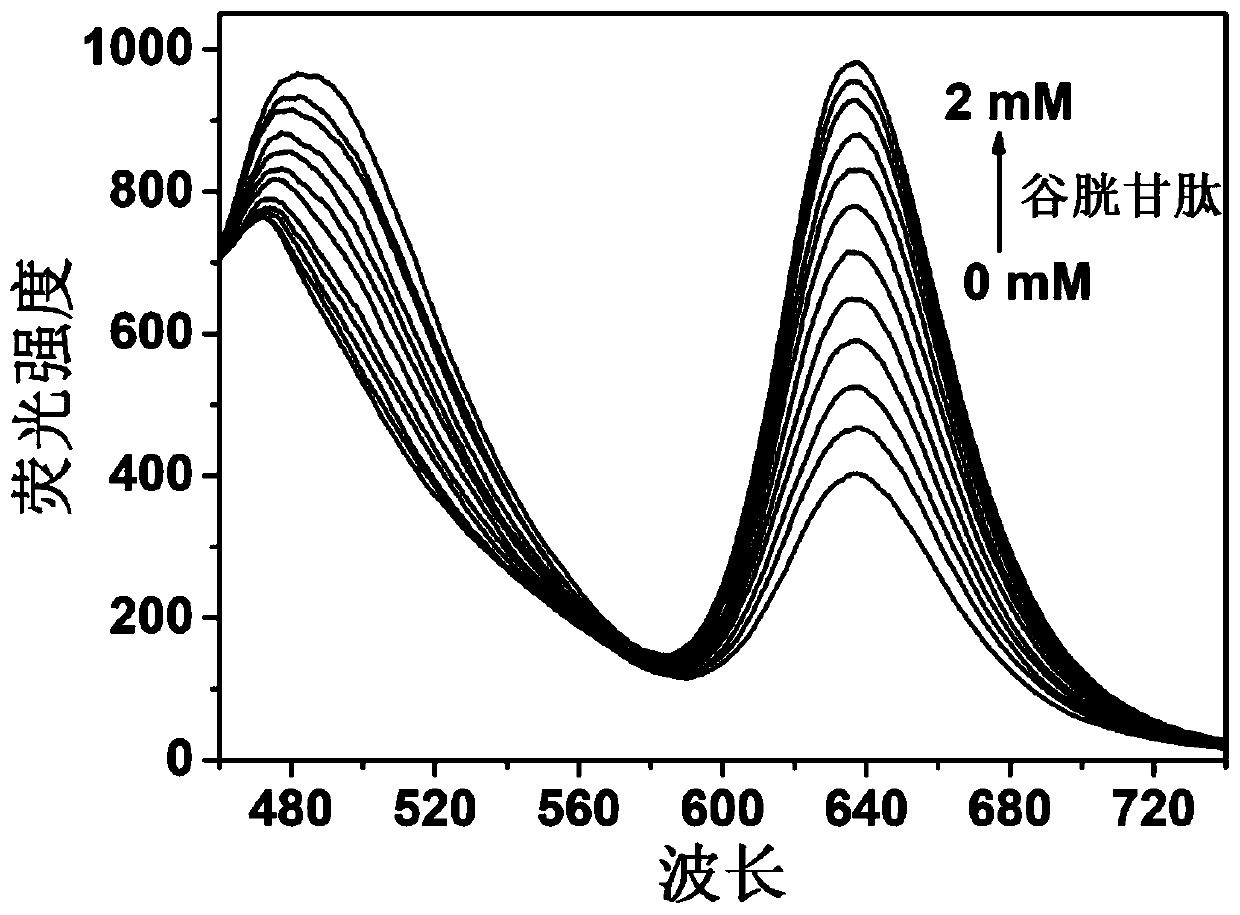
[0053] Prepare a PBS buffer solution with a pH of 7.4 and a concentration of 10 mM, prepare a 0.2 M glutathione solution, dissolve CM-BP in DMSO to prepare a 2 mM solution; take 2 mL of a PBS solution containing 10% DMSO at a pH of 7.4, Add 10 μL DMSO solution of CM-BP into a fluorescence cuvette, add glutathione to the fluorescence cuvette, with the addition of glutathione to be tested, the fluorescence intensity at 638nm gradually increases; the fluorescence emission picture see Figure 4 .
Embodiment 3
[0055] Prepare a PBS buffer solution with a pH of 7.4 and a concentration of 10mM, prepare a 0.2M glutathione solution, dissolve CM-BP in DMSO to prepare a 2mM solution; add 2mL of 10% to each of the 10 cuvettes DMSO pH 7.4 PBS solution, 10 μL CM-BP DMSO solution, add glutathione solution to the cuvette with volumes of 0, 2, 4, 6, 8, 10, 12, 14, 16, 18 , 20 μL, and then measure the fluorescence intensity at 638nm on the fluorescence spectrometer as 402, 469, 525, 588, 648, 713, 777, 826, 879, 925, 953. Take the concentration of glutathione as the abscissa and the fluorescence intensity as the ordinate to draw a graph to obtain the working curve of the concentration of glutathione; the linear regression equation is: F 638 =283.9c+416.5, the unit of c is 10 -3 mol / L; (see Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com