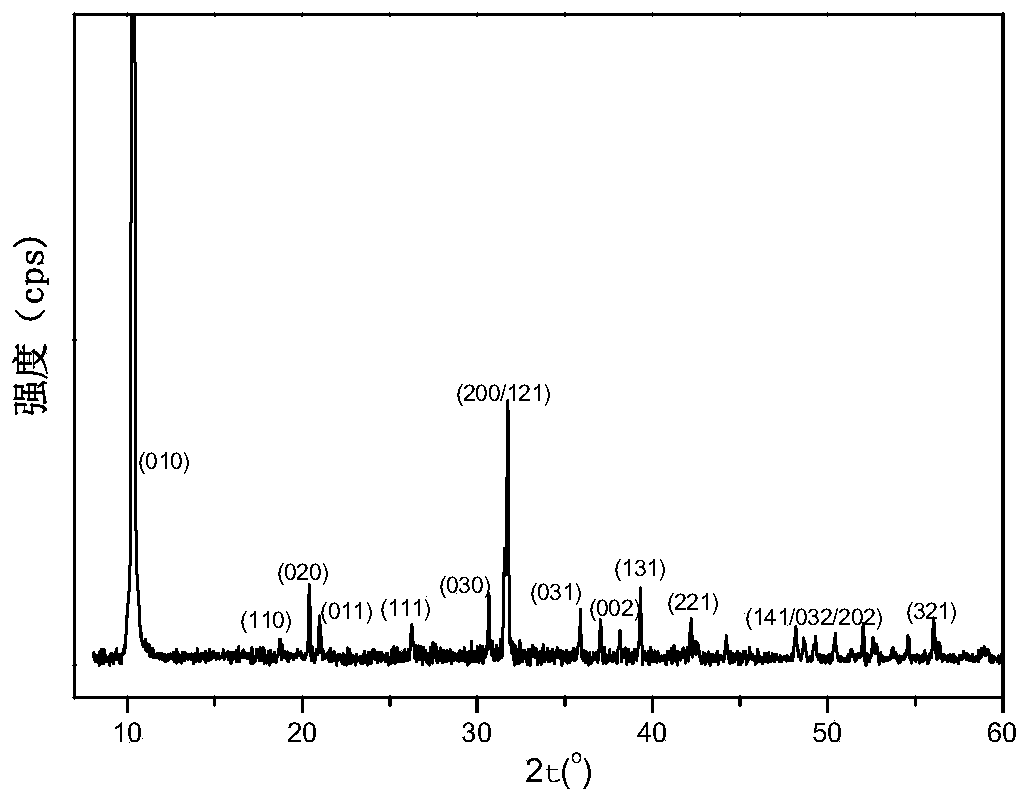
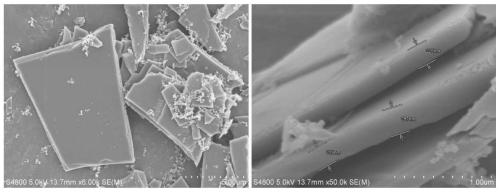
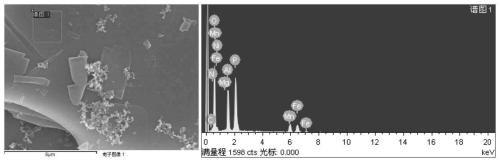
Ferromanganese phosphate intermediate, lithium ferromanganese phosphate, and method for producing same
A technology of lithium manganese iron phosphate and intermediates, applied in lithium manganese iron phosphate, lithium manganese iron phosphate in the field of lithium batteries, can solve the problem of reducing the volume energy ratio of materials, low compaction density of lithium manganese iron phosphate, and difficulty in obtaining synthetic methods Theoretical measurement ratio and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] According to the molar ratio of Mn:Fe=50:50, the content of manganese sulfate and ferrous sulfate is 10g / L (with MnSO 4 ·H 2 O and FeSO 4 ·7H 2 O (meter) of the aqueous solution, a total of 5500 mL, the equivalent metal molar weight is 0.246 mol, denoted as liquid A-1.
[0110] Using 30g 85% industrial phosphoric acid as the phosphorus source (0.26mol), using the sulfuric acid with a concentration of 1M as the pH control reagent, and about 33.2g 26%wt ammonia water as the nitrogen source (0.247mol), under stirring, liquid A-1 , industrial phosphoric acid and ammonia water are simultaneously added into the reaction kettle whose temperature is room temperature ± 5 ℃, and the pH is controlled to be 3.0 ± 0.2 by regulating the adding speed of sulfuric acid. After the addition of materials, the reaction was completed by stirring at a constant temperature for 24 hours.
[0111] The precipitate obtained above was filtered, washed with deionized water, and then dried in an ...
Embodiment 2
[0116] According to the molar ratio of Mn:Fe=80:20, an aqueous solution of manganese chloride (tetrahydrate) and ferrous chloride (tetrahydrate) with a content of 200g / L was prepared, a total of 3000mL, and the equivalent metal molar weight was 3.03mol, denoted as Liquid Armor-2.
[0117] Take 350g 85% phosphoric acid (3.03mol) as the phosphorus source, take the 2kg ammonium sulfate (3.03mol) aqueous solution with a concentration of 20%wt as the nitrogen source, and take the sodium hydroxide with a concentration of 10M as the pH control reagent, under stirring, the Liquid A-2, phosphoric acid and ammonium sulfate solution were simultaneously added to the reaction kettle with a temperature range of 70±5°C, and the pH was controlled to be 5.5±0.2 by regulating the addition rate of sodium hydroxide. After the addition was completed, the temperature was kept constant for 12 hours to complete the reaction.
[0118] The precipitate obtained above was filtered, washed with deionized...
Embodiment 3
[0123] According to the molar ratio of Mn:Fe=7:1, an aqueous solution with a content of manganese chloride (tetrahydrate) and ferrous chloride (tetrahydrate) of 200g / L was prepared, a total of 3000mL, and the equivalent metal molar amount was 3.03mol, which was recorded as Liquid Armor-3.
[0124] With 350g 85% phosphoric acid (3.03mol) as phosphorus source, with concentration of 1.2kg ammonium bicarbonate (3.03mol) aqueous solution of 20%wt as nitrogen source, with concentration of 1M sodium hydroxide as pH control reagent, under stirring , the liquid methyl-2, phosphoric acid, and ammonium bicarbonate solution were simultaneously added to the reaction kettle with a temperature range of 70±5°C, and the pH was controlled to be 5.5±0.2 by regulating the adding speed of sodium hydroxide. After the addition was completed, the temperature was kept constant for 12 hours to complete the reaction.
[0125] The precipitate obtained above was filtered, washed with deionized water, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com