Construction method and application of attenuated Listeria monocytogenes
A Listeria and monocyte technology, applied in the field of genetic engineering, can solve the problems of easy transfer and recurrence, long immunization cycle, poor immunization effect, etc., to eliminate plasmid loss, stable attenuated strains, and good immunogenicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The construction of embodiment 1 attenuated vaccine vector
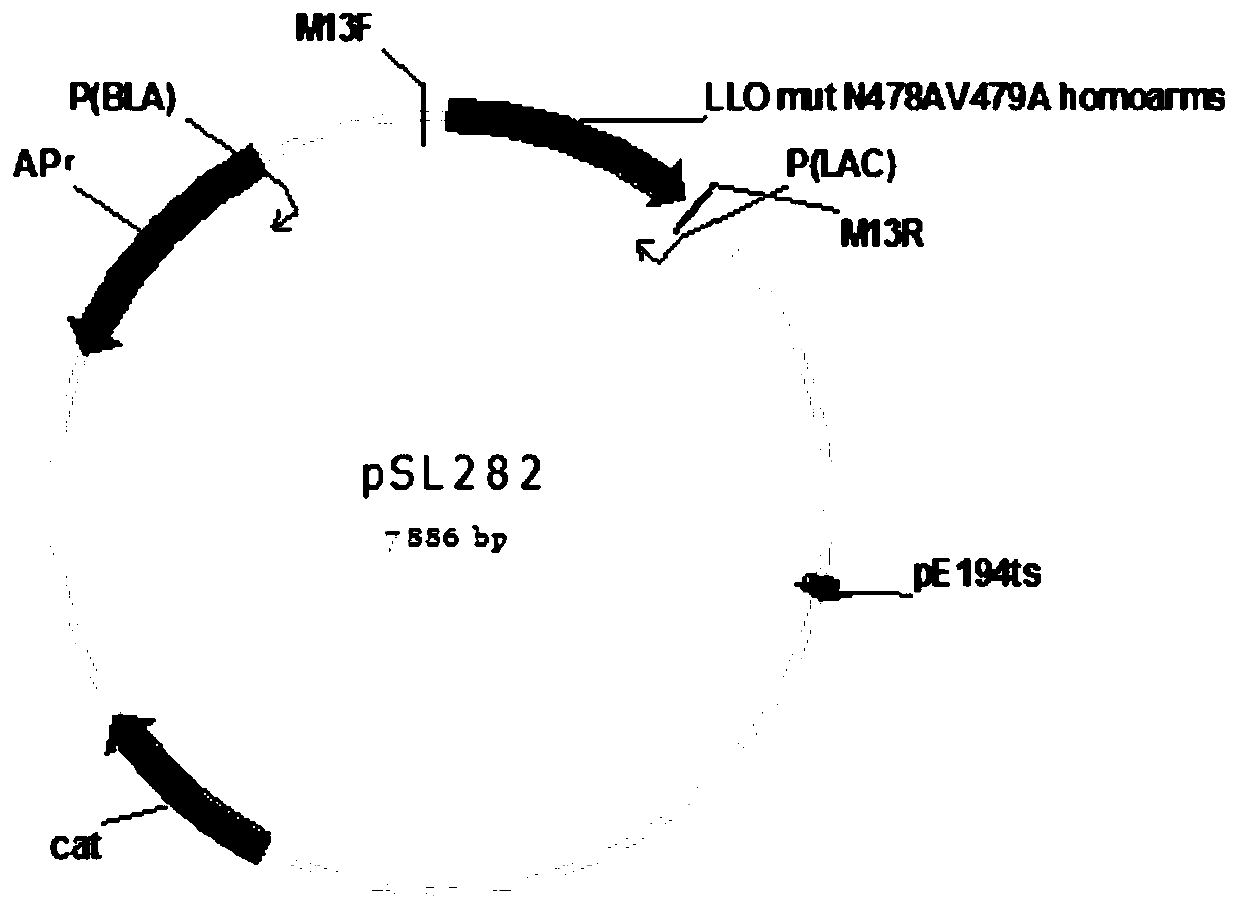
[0051] 1. Construction of recombinant plasmids
[0052] From Listeria monocytogenes wild strain EGD-e bacterial strain (ATCC standard bacterial strain), directly clone required fragment, use Vector NTI Explorer to design amplification primer pSL279-Sal I-F and pSL279-BamH I-R, full-length 819bp (as SEQ ID NO .1), related primers are shown in Table 1:
[0053] Table 1 Primers required for gene amplification and verification of EGD-e hly
[0054]
[0055] Note: pSL279-Sal I-F is the upstream primer, pSL279-BamH I-R is the downstream primer, homoarms front is the primer used for verification at a distance of 63bp from the homology arm on the Listeria genome, M13-F is the upstream verification primer on the pKSV7 plasmid, M13 -R is the downstream verification primer on the pKSV7 plasmid, pSL282-F is the upstream primer for constructing the spike, and pSL282-R is the downstream primer for constructing the spik...
Embodiment 2
[0063] Example 2 Attenuated strain phenotype analysis and infection biology analysis
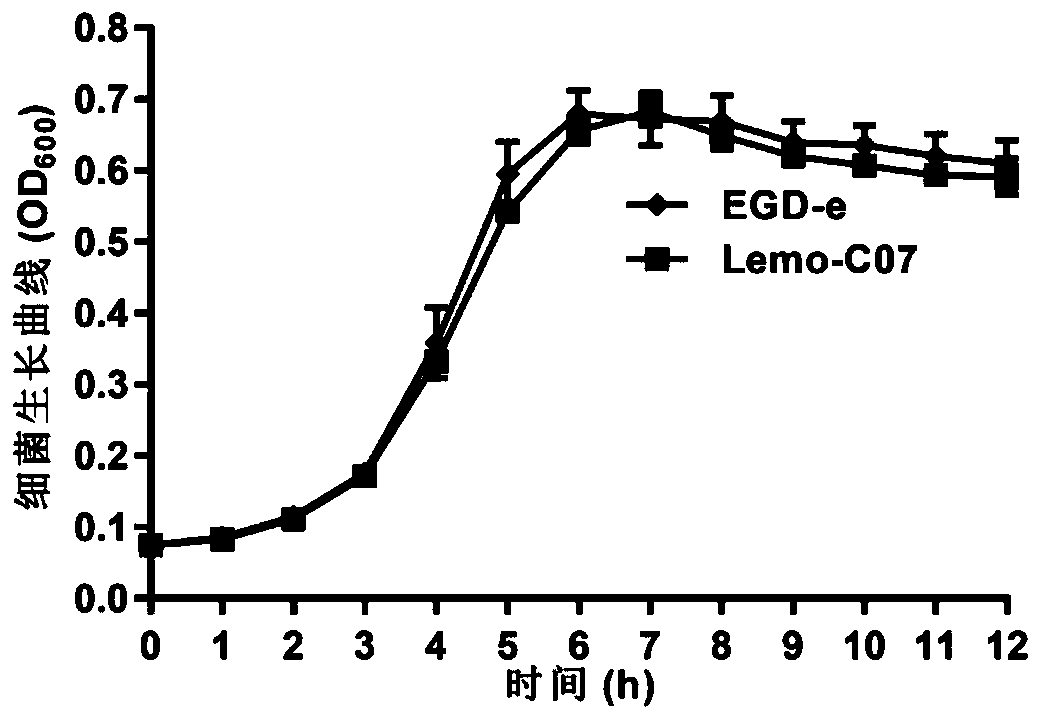
[0064] 1. Growth ability analysis
[0065] Pick a single colony in 5ml of BHI liquid medium, place it in a shaker at 37°C for overnight culture, take 1ml of the bacterial solution to adjust the OD to 0.2, then dilute it 100 times with fresh BHI medium, take 200μl in a 96-well enzyme plate , 3 parallels for each bacterium, measure the optical density value (OD value) at 600nm with a microplate reader, place it in a constant temperature incubator at 37°C, measure once every 1h, and measure continuously for 12h. Depend on image 3 As shown, compared with the wild strain EGD-e, the growth ability of Lemo-C07 in BHI medium was not affected.
[0066] 2. LLO and its mutant LLO N478AV479A Protein expression assay
[0067] Pick a single colony in 5ml BHI liquid medium, place it on a shaker at 37°C for overnight culture, take 1ml of the bacterial liquid and transfer it to 100ml BHI liquid medium, ...
Embodiment 3
[0078] Immune effect evaluation of embodiment 3 attenuated strain
[0079] 1. Determination of transcriptional levels of immune factors in mouse macrophages
[0080] Plate Raw264.7 cells in a 6-well cell culture plate, culture overnight, infect the cells with Listeria at MOI=10:1, use PBS as a blank control, incubate at 37°C for 30min, and wash with 10mM PBS (pH 7.4) 2-3 times, add DMEM containing 50 μg / ml gentamicin to continue culturing, wash 2-3 times with 10 mM PBS (pH 7.4) after 30 min, add DMEM containing 10% FBS and 5 μg / ml gentamicin to continue Cultivate for 3h and 6h, digest the cells with 0.25% trypsin at each time point, centrifuge, take the cells, extract cellular RNA with a total cellular RNA extraction kit, and reverse into cDNA with a reverse transcription kit, RT-PCR Detect relevant inflammatory factors, relevant primers are shown in Table 2:
[0081] Table 2 Primers required for qPCR
[0082]
[0083] Note: F stands for upstream primer, R stands for dow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com