Method for preparing 4-ethylphenol from glycerol
A technology of ethyl phenol and glycerol, applied in the field of bioengineering, can solve problems such as unfavorable environment, no discovery of 4-ethyl phenol metabolic pathway, complicated process, etc., and achieves less environmental pollution, important economic value and social benefit, and mild conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Construction of Escherichia coli recombinant plasmid pETDuet-tal-pad-vpr
[0036] The primer sequences are shown in Table 1, where the underlined part is the restriction site.
[0037] Table 1 The primers used in this embodiment
[0038]
[0039]
[0040] The recombinant plasmid pETDuet-tal-pad-vpr contains three genes, namely setal, bapad and lpvpr, each gene is controlled by an independent T7 promoter and sugar body binding site RBS, the main construction steps are as follows:
[0041] 1) Using pET21b-setal as a template, using setal-F and setal-R as primers, perform PCR amplification on the sequence of setal. The setal-PCR fragment was cloned into the vector pETDuet-1, and the restriction sites were SacI and NotI to generate pETDuet-setal.
[0042] 2) Using pET28a-bapad as a template and using bapad-F and bapad-R as primers to perform PCR amplification on the sequence of bapad. The bapad-PCR fragment was digested with NdeI and XhoI and cloned into ...
Embodiment 2
[0045] Expression and purification analysis of three kinds of enzymes in the recombinant bacterial strain of embodiment 2
[0046] The constructed recombinant plasmid pETDuet-tal-pad-vpr was transformed into Escherichia coli competent E.coli TransB(DE3) to obtain the recombinant strain E.coli TransB-TPV. Recombinant bacteria were cultured overnight at 37°C in 4 mL of LB medium, then inoculated into 50 mL of LB medium at a 2% inoculum size, and osmotic agents (1M sorbitol and 2.5mM betaine) were added to the medium to increase enzyme activity. Soluble expression. Cultured at 37℃ to OD 600 When the temperature was 0.8, IPTG with a final concentration of 0.2mM was added, and the cells were collected after induction at 20°C for 16h. The collected cells were centrifuged at 8,000 rpm for 10 min at 4°C, the supernatant was removed, and 5 mL of Lysis buffer (50 mM NaH 2 PO 4 , 300mM NaCl, pH8.0), and vortexed to mix evenly. Then, the cells were disrupted with an ultrasonic disrup...
Embodiment 3
[0048] Shake flask fermentation culture of embodiment 3 recombinant strains
[0049] (1) Effect of host bacteria and penetrant on the production of 4-ethylphenol
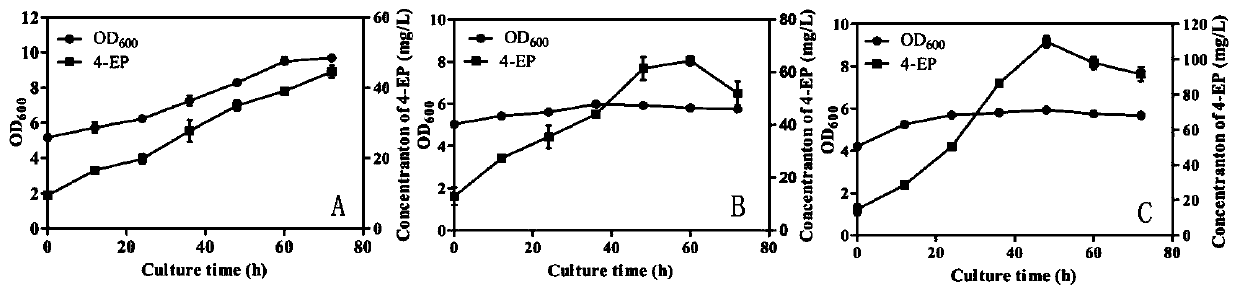
[0050] The recombinant plasmid pETDuet-tal-pad-vpr was transformed into Escherichia coli E.coli BL21(DE3) and E.coliTransB(DE3) respectively to obtain recombinant bacteria E.coli BL21-TPV and E.coli TransB-TPV. The recombinant bacteria were cultured overnight at 37°C in 4 mL of LB medium, and then inserted into 100 mL of TB medium at an inoculum size of 2%. The TB medium was divided into two parts, one with osmotic agent added (1 M sorbitol and 2.5 mM betaine) and the other without osmotic agent added. Cultured at 37℃ to OD 600 When it is 0.8, add IPTG with a final concentration of 0.2mM, and culture at 20°C for 72h. The concentration of product 4-ethylphenol was measured by sampling every 12h.
[0051] Such as figure 2 As shown, after shaking the flask for 72 hours, the 4-ethylphenol production of the recombi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com