Synthesis method of aminophenol
A technology of aminophenol and synthesis method, which is applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of increased post-processing difficulty, large amount of three wastes, poor process safety, etc., and achieves the difficulty of three-waste treatment. Small, high yield and purity, simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
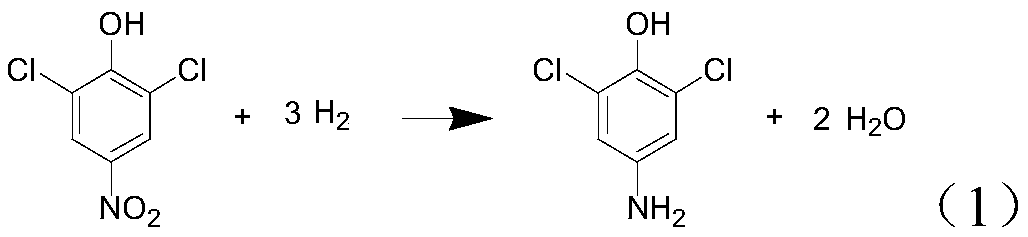
[0078] The present embodiment provides a kind of synthetic method of 2,6-dichloro-4-aminophenol, and described method comprises the steps:
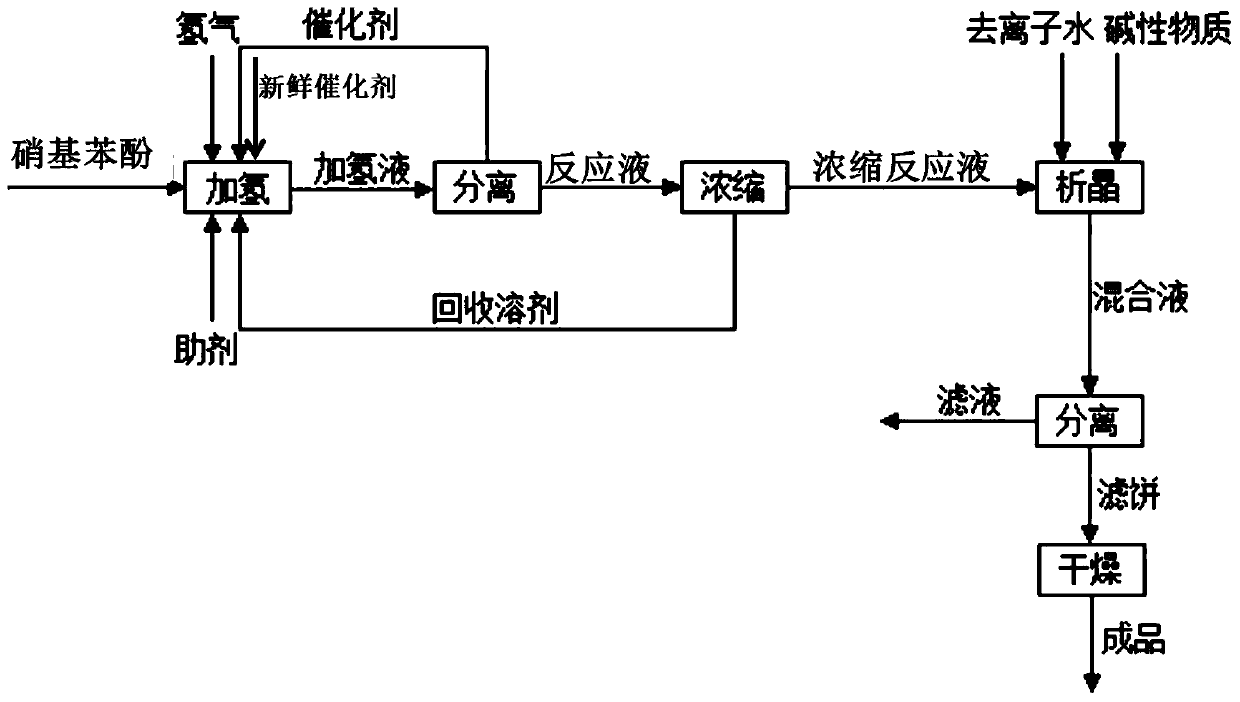
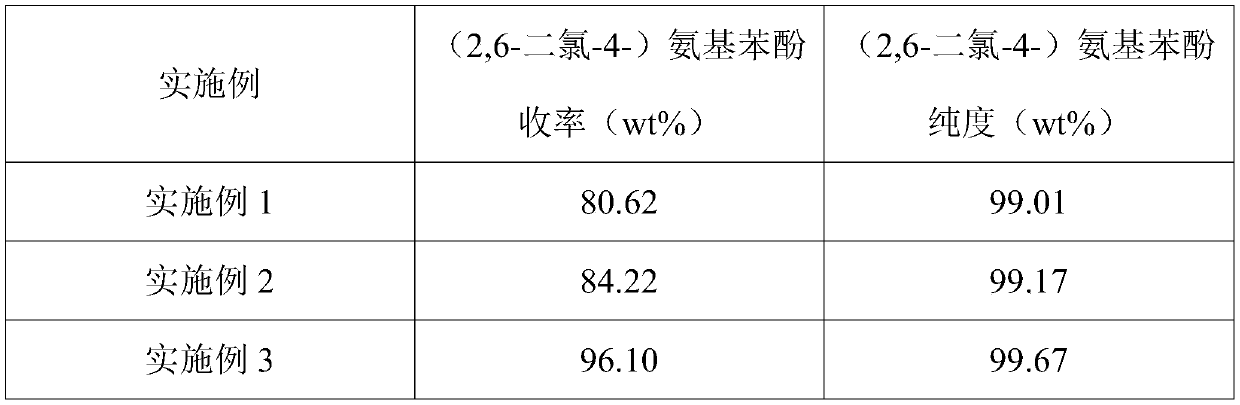
[0079] (1) Put 2,6-dichloro-4-nitrophenol and tetrahydrofuran into the autoclave, wherein the mass ratio of 2,6-dichloro-4-nitrophenol and tetrahydrofuran is 0.01:1, Rh / Al 2 o 3 Supported catalyst and auxiliary agent acetic acid, wherein the mass ratio of catalyst to 2,6-dichloro-4-nitrophenol is 0.01:1, and the molar ratio of auxiliary agent acetic acid to 2,6-dichloro-4-nitrophenol is 2:1; carry out the hydrogenation reaction at a reaction temperature of 30°C and a pressure of 0.2MPa for a reaction time of 10 hours. After the reaction is completed, the temperature is lowered to release the pressure, and the reaction liquid and the catalyst are separated, and the catalyst is returned to the hydrogenation reaction for use;
[0080] (2) The reaction solution after separation is subjected to negative pressure distillation under an absolute...
Embodiment 2
[0083] The present embodiment provides a kind of synthetic method of 2,6-dichloro-4-aminophenol, and described method comprises the steps:
[0084](1) Put 2,6-dichloro-4-nitrophenol and ethanol into the autoclave, wherein the mass ratio of 2,6-dichloro-4-nitrophenol to ethanol is 0.8:1, Raney nickel Catalyst and auxiliary agent sulfuric acid, wherein the mass ratio of Raney nickel catalyst to 2,6-dichloro-4-nitrophenol is 0.1:1, and the molar ratio of auxiliary agent sulfuric acid to 2,6-dichloro-4-nitrophenol The ratio is 0.01:1; the hydrogenation reaction is carried out at a reaction temperature of 40°C and a pressure of 0.5 MPa, and the reaction time is 5 hours. After the reaction is completed, the temperature is lowered and the pressure is released, and the reaction liquid and the catalyst are separated, and the catalyst is returned to the hydrogenation reaction for mechanical application;
[0085] (2) The reaction solution after separation is carried out negative pressure...
Embodiment 3
[0088] The present embodiment provides a kind of synthetic method of 2,6-dichloro-4-aminophenol, and described method comprises the steps:
[0089] (1) Put 2,6-dichloro-4-nitrophenol and methanol into the autoclave, wherein the mass ratio of 2,6-dichloro-4-nitrophenol to methanol is 0.3:1, Pd / C Supported catalyst and auxiliary agent hydrochloric acid, wherein the mass ratio of catalyst to 2,6-dichloro-4-nitrophenol is 0.05:1, and the molar ratio of auxiliary agent hydrochloric acid to 2,6-dichloro-4-nitrophenol is 0.1:1; carry out the hydrogenation reaction at a reaction temperature of 50°C and a pressure of 1 MPa, and the reaction time is 2 hours. After the reaction is completed, the temperature is lowered and the pressure is released, and the reaction liquid and the catalyst are separated, and the catalyst is returned to the hydrogenation reaction for mechanical application;
[0090] (2) The reaction solution after separation is carried out under the absolute pressure 5kPa t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com